Abstract
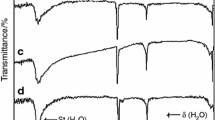
Stages 4-6 FeCl3-graphite intercalation compounds (GIC’s) have been prepared by an ordinary two-bulb method, and FeCl3-IBr-graphite bi-intercalation compounds (GBC’s) are synthesized by holding the FeCl3-GIC’s in the saturated vapor of IBr. The x-ray diffraction patterns of the FeCl3-IBr-GBC’s obtained from stages 4, 5, and 6 FeCl3-GIC’s give the stacking sequences as G(FeCl3)GG(IBr)GG(FeCl3)G, G(FeCl3)GG(IBr)GGG(FeCl3)G, and G(FeCl3)GG(IBr)GG(IBr)GG(FeCl3)G, respectively, where G, (FeCl3), and (IBr) refer to the graphite, FeCl3, and IBr layers, respectively. The multi-intercalation of H2SO4 into the FeCl3-IBr-GBC’s synthesized from stages 4 and 6 FeCl3-GIC’s occurs at all the vacant galleries of the GBC’s at the same time. In contrast, the multi-intercalation of H2SO4 into the FeCl3-IBr-GBC obtained from the stage 5 FeCl3-GIC takes place in two processes. The first multi-intercalation occurs at the gallery adjacent to the bi-intercalated IBr layer, and the stacking sequence of the resulting graphite multi-intercalation compound is determined to be G(FeCl3)GG(IBr)G(H2SO4)GG(FeCl3)G, where (H2SO4) refers to the H2SO4 layer. The second multi-intercalation occurs at all the rest of the vacant galleries.
Similar content being viewed by others
References
P. Lagrange, A. Métrot, and A. Hérold, C. R. Acad. Sci. Paris Ser. C 273, 701 (1974).
A. G. Freeman, J. C. S. Chem. Comm., 746 (1974).
P. Scharff, E. Stumpp, and C Ehrhardt, Synth. Met. 23, 415 (1988).
B. R. York, S. K. Hark, and S. A. Solin, Synth. Met. 7, 25 (1983).
A. Hérold, G. Furdin, D. Guérard, L. Hachim, M. Lelaurain, N. E. Nadi, and R. Vangelisti, Synth. Met. 12, 11 (1985).
N. E. Nadi, E. McRae, J. F. Marêché, M. Lelaurain, and A. Hérold, Carbon 24, 695 (1986).
M. Suzuki, P. C. Chow, and H. Zabel, Phys. Rev. B 32, 6800 (1985).
H. Shioyama, K. Tatsumi, R. Fujii, and Y. Mizutani, Carbon 28, 119 (1990).
Y. Mizutani, T. Abe, M. Asano, and T. Harada, J. Mater. Res. 8, 1586 (1993).
B. D. Cullity, X-Ray Diffraction (Addison-Wesley, Reading, MA, 1956).
International Tables for X-ray Crystallography III (The Kynoch Press, Birmingham, U. K., 1962), p. 210.
J. M. Cowley and J. A. Iber, Acta Crystallogr. 9, 421 (1956).
W. Rüdorff, Z. Phys. Chem. 45, 2 (1940).
W. Metz and D. Hohlwein, Carbon 13, 87 (1975).
Y. Mizutani, Thesis, Kyoto University (1985).
H. Mazurek, G. Ghavamishahidi, G. Dresselhaus, and M. S. Dresselhaus, Carbon 20, 415 (1982).
G. Colin and A. Hérold, C. R., 244, 2294 (1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abe, T., Mizutani, Y., Ihara, E. et al. Preparation of FeCl3–IBr–H2SO4–graphite multi-intercalation compounds. Journal of Materials Research 9, 377–382 (1994). https://doi.org/10.1557/JMR.1994.0377
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.1994.0377