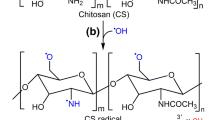
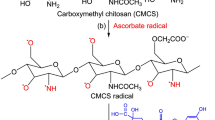
Abstract
Since chitosan and its amino-, cinnamo- or cinnamo-amino- derivatives are acid-soluble, the effect of acetic acid on hyaluronan (HA) macromolecules degraded by Cu(II) ions and ascorbate was examined to produce reactive oxygen species (ROS). Further, the effects of glutathione (GSH), chitosan and its derivatives, added individually or in combination, on the quenching of ROS and ABTS˙+ cation radical were examined using rotational viscometry and ABTS assay, respectively. The results of the rotational viscometry indicated a rapid degradation of HA by ROS after the addition of acetic acid. Chitosan and its derivatives moderately decreased the rate of HA degradation, while GSH decreased the rate of HA degradation more significantly. Moreover, GSH enhanced the protection of HA macromolecules against their degradation in the presence of chitosan or its derivatives. The results of the ABTS assay confirmed the results of the rotational viscometry. The GSH in the combination with chitosan and its derivatives reduced ABTS˙+ more intensively than when added individually.
Similar content being viewed by others
References
Aya, K. L., & Stern, R. (2014). Hyaluronan in wound healing: Rediscovering a major player. Wound Repair and Regeneration, 22, 579–593. DOI: 10.1111/wrr.12214.
Banasova, M., Valachova, K., Juranek, I., & Šoltés, L. (2014). Dithiols as more effective than monothiols in protecting biomacromolecules from free-radical-mediated damage: in vitro oxidative degradation of high-molar-mass hyaluronan. Chemical Papers, 68, 1428–1434. DOI: 10.2478/s11696–014-0591–1.
Cyphert, J. M., Trempus, C. S., & Garantziotis, S. (2015). Size matters: Molecular weight specificity of hyaluronan effects in cell biology. International Journal of Cell Biology, 2015, article ID 563818. DOI: 10.1155/2015/563818.
Demirkol, O., Adams, C., & Ercal, N. (2004). Biologically important thiols in various vegetables and fruits. Journal of Agricultural and Food Chemistry, 52, 8151–8154. DOI: 10.1021/jf040266f.
Dodane, V., & Vilivalam, V. D. (1998). Pharmaceutical applications of chitosan. Pharmaceutical Science & Technology Today, 1, 246–253. DOI: 10.1016/s1461–5347(98)00059–5.
Gigante, A., & Callegari, L. (2011). The role of intra-articular hyaluronan (Sinovial®) in the treatment of osteoarthritis. Rheumatology International, 31, 427–444. DOI: 10.1007/s00296–010–1660–6.
Haddad, J. J., & Harb, H. L. (2005). L-γ-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Molecular Immunology, 42, 987–1014. DOI: 10.1016/j.molimm.2004.09.029.
Hami, Z., Amini, M., Kiani, A., & Ghazi-Khansari, M. (2013). High performance liquid chromatography coupled with precolumn derivatization for determination of oxidized glutathione level in rats exposed to paraquat. Iranian Journal of Pharmaceutical Reseach, 12, 911–916.
Islam, M. M., Masum, S. M., Rahman, M. M., Molla, M. A. I., A. A., & Roya, S. K. (2011). Preparation of chitosan from shrimp shell and investigation of its properties. International Journal of Basic and Applied Science IJBAS-IJENS, 11, 77–80.
Jeon, Y. J., Shahidi, F., & Kim, S. K. (2000). Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Reviews International, 16, 159–176. DOI: 10.1081/fri-100100286.
Kujawa, P., Moraille, P., Sanchez, J., Badia, A., & Winnik, F. M. (2005). Effect of molecular weight on the exponential growth and morphology of hyaluronan/chitosan multilayers: A surface plasmon resonance spectroscopy and atomic force microscopy investigation. Journal of the American Chemical Society, 127, 9224–9234. DOI: 10.1021/ja044385n.
Kumar, B. A. V., Varadaraj, M. C., & Tharanathan, R. N. (2007). Low molecular weight chitosan — preparation with the aid of pepsin, characterization, and its bactericidal activity. Biomacromolecules, 8, 566–572. DOI: 10.1021/bm060753z.
Lim, S. T., Forbes, B., Martin, G. P., & Brown, M. B. (2001). In vivo and in vitro characterization of novel microparticulates based on hyaluronan and chitosan hydroglutamate. AAPS Pharm Sci Tech, 2, article 20. DOI: 10.1007/bf02830560.
Mohy Eldin, M. S., Soliman, E. A., Hashem, A. I., & Tamer, T. M. (2012). Antimicrobial activity of novel aminated chitosan derivatives for biomedical applications. Advances in Polymer Technology, 31, 414–428. DOI: 10.1002/adv.20264.
Mohy Eldin, M. S., Hashem, A. I., Omer, A. M., & Tamer, T. M. (2015). Preparation, characterization and antimicrobial evaluation of novel cinnamyl chitosan Schiff base. International Journal of Advanced Research, 3, 741–755.
Necas, J., Bartosikova, L., Brauner, P., & Kolar, J. (2008). Hyaluronic acid (hyaluronan): a review. Veterinární Medicína, 53, 397–411.
Oyarzun-Ampuero, F. A., Brea, J., Loza, M. I., Torres, D., & Alonso, M. J. (2009). Chitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. International Journal of Pharmaceutics, 381, 122–129. DOI: 10.1016/j.ijpharm.2009.04.009.
Papakonstantinou, E., Roth, M., & Karakiulakis, G. (2012). Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology, 4, 253–258. DOI: 10.4161/derm.21923.
Rah, M. J. (2011). A review of hyaluronan and its ophthalmic applications. Optometry — Journal of the American Optometric Association, 82, 38–43. DOI: 10.1016/j.optm.2010.08.003.
Rapta, P., Valachova, K., Gemeiner, P., & Šoltés, L. (2009). High-molar-mass hyaluronan behavior during testing its radical scavenging capacity in organic and aqueous media: Effects of the presence of manganese(II) ions. Chemistry & Biodiversity, 6, 162–169. DOI: 10.1002/cbdv.200800075.
Rees, M. D., Kennett, E. C., Whitelock, J. M., & Davies, M. J. (2008). Oxidative damage to extracellular matrix and its role in human pathologies. Free Radical Biology & Medicine, 44, 1973–2001. DOI: 10.1016/j.freeradbiomed.2008.03.016.
Reitinger, S., & Lepperdinger, G. (2013). Hyaluronan, a ready choice to fuel regeneration: A mini-review. Gerontology, 59, 71–76. DOI: 10.1159/000342200.
Rigby, G. W. (1936). U.S. Patent No. 2040879. Washington, D. C., USA: U.S. Patent and Trademark Office.
Sezer, A. D., Hatipoğlu, F., Cevher, E., Ogurtan, Z., Bas, A. L., & Akbuğa, J. (2007). Chitosan film containing fucoidan as a wound dressing for dermal burn healing: Preparation and in vitro/in vivo evaluation. AAPS PharmSciTech, 8, E94–E101. DOI: 10.1208/pt0802039.
Shahidi, F., Arachchi, J. K. V., & Jeon, Y. J. (1999). Food applications of chitin and chitosans. Trends in Food Science & Technology, 10, 37–51. DOI: 10.1016/s0924–2244(99)00017–5.
Signini, R., & Campana Filho, S. P. (1999). On the preparation and characterization of chitosan hydrochloride. Polymer Bulletin, 42, 159–166. DOI: 10.1007/s002890050448.
Šoltés, L., Stankovska, M., Brezova, V., Schiller, J., Arnhold, J., Kogan, G., & Gemeiner, P. (2006). Hyaluronan degradation by copper(II) chloride and ascorbate: rotational viscometric, EPR spin-trapping, and MALDI-TOF mass spectrometric investigations. Carbohydrate Research, 341, 2826–2834. DOI: 10.1016/j.carres.2006.09.019.
Šoltés, L., Tamer, M. T., Veverka, M., Valachova, K., & Mohy Eldin, M. S. (2015). SK Patent Application No. PP 50322015. Banska Bystrica: Industrial Property Office of the Slovak Republic.
Stern, R., & Maibach, H. I. (2008). Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clinics in Dermatology, 26, 106–122. DOI: 10.1016/j.clindermatol.2007.09.013.
Tan, H., Chu, C. R., Payne, K. A., & Marra, K. G. (2009). Injectable in situ forming biodegradable chitosan—hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials, 30, 2499–2506. DOI: 10.1016/j.biomaterials.2008.12. 080.
Topolska, D., Valachova, K., Rapta, P., Silhar, S., Panghyova, E., Horvath, A., & Šoltés, L. (2015). Antioxidative properties of Sambucus nigra extracts. Chemical Papers, 69, 1202–1210. DOI: 10.1515/chempap-2015–0138.
Valachova, K., Vargova, A., Rapta, P., Hrabarova, E., Drafi, F., Bauerova, K., Juranek, I., & Šoltés, L. (2011). Aurothiomalate as preventive and chain-breaking antioxidant in radical degradation of high-molar-mass hyaluronan. Chemistry & Biodiversity, 8, 1274–1283. DOI: 10.1002/cbdv.201000351.
Van den Bekerom, M. P. J., Mylle, G., Rys, B., & Mulier, M. (2006). Viscosupplementation in symptomatic severe hip osteoarthritis: A review of the literature and report on 60 patients. Acta Orthopaedica Belgica, 72, 560–568.
Wolfrom, M. L., Maher, G. G., & Chaney, A. (1958). Chitosan nitrate. The Journal of Organic Chemistry, 23, 1990–1991. DOI: 10.1021/jo01106a049.
Xing, G., Ren, M., & Verma, A. (2014). Divergent temporal expression of hyaluronan metabolizing enzymes and receptors with craniotomy vs. controlled-cortical impact injury in rat brain: a pilot study. Frontiers in Neurology, 5, article number 173. DOI: 10.3389/fneur.2014.00173.
Yamane, S., Iwasaki, N., Majima, T., Funakoshi, T., Masuko, T., Harada, K., Minami, A., Monde, K., & Nishimura, S. (2005). Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterials, 26, 611–619. DOI: 10.1016/j.biomaterials.2004.03.013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valachová, K., Tamer, T.M., Eldin, M.M. et al. Radical-scavenging activity of glutathione, chitin derivatives and their combination. Chem. Pap. 70, 820–827 (2016). https://doi.org/10.1515/chempap-2016-0011
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2016-0011