Abstract
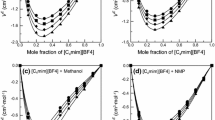
Thermophysical properties, such as density (ρ), refractive index (nD), and viscosity (η) of the binary systems of ionic liquids (ILs) [C n mim]Cl (C n mim = 1-alkyl-3-methylimidazolium; n = 2, 4) and three dipolar aprotic solvents (NMP (N-methyl-2-pyrrolidinone), DMI (1,3-dimethyl-2-imidazolidinone), and DMPU (1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone)) were measured based on the solubility of ILs in the molecular solvents at temperatures from 288.15 K to 318.15 K and the pressure of p = 0.1 MPa. Moreover, intermolecular interactions, such as weak hydrogen bonding and Coulomb forces, were discussed based on the determined properties.
Similar content being viewed by others
Abbreviations
- Ai:
-
cubic equation parameters
- N:
-
the number of experimental points
- n:
-
the number of carbon atoms
- n D :
-
refractive index
- p:
-
pressure Pa
- RSD:
-
Relative standard deviation %
- T:
-
temperature K, °C
- xi:
-
the molar fraction of component i
- x̄:
-
mean value
- Y:
-
variable
- ε:
-
dielectric constant
- η:
-
viscosity mPa s
- μ:
-
dipole moment D
- ρ:
-
density g cm−3
- σ:
-
standard deviation
References
Blahut, A., Dohnal, V., & Vrbka, P. (2012). Interactions of volatile organic compounds with the ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate. The Journal of Chemical Thermodynamics, 47, 100–108. DOI: 10.1016/j.jct.2011.09.028.
Canongia Lopes, J. N., Costa Gomes, M. F., Husson, P., Pádua, A. A. H., Rebelo, L. P. N., Sarraute, S., & Tariq, M. (2011). Polarity, viscosity, and ionic conductivity of liquid mixtures containing [C4C1im][Ntf2] and a molecular component. The Journal of Physical Chemistry B, 115, 6088–6099. DOI: 10.1021/jp2012254.
Ferreira, A. R., Freire, M. G., Ribeiro, J. C., Lopes, F. M., Crespo, J. G., & Coutinho, J. A. P. (2012). Overview of the liquid-liquid equilibria of ternary systems composed of ionic liquid and aromatic and aliphatic hydrocarbons, and their modeling by COSMO-RS. Industrial & Engineering Chemistry Research, 51, 3483–3507. DOI: 10.1021/ie2025322.
Fic, K., Lota, G., Meller, M., & Frackowiak, E. (2012). Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy & Environmental Science, 5, 5842–5850. DOI: 10.1039/c1ee02262h.
García-Giménez, P., Gil, L., Blanco, S. T., Velasco, I., & Otín, S. (2007). Densities and isothermal compressibilities at pressures up to 20 MPa of the systems 1-methyl-2-pyrrolidone+ 1-chloroalkane or + α,ω-dichloroalkane. Journal of Chemical & Engineering Data, 53, 66–72. DOI: 10.1021/je7003758.
Greaves, T. L., & Drummond, C. J. (2008). Protic ionic liquids: Properties and applications. Chemical Reviews, 108, 206–237. DOI: 10.1021/cr068040u.
Ivanov, E. V., & Lebedeva, E. Y. (2009). D2O—H2Osolvent isotope effects on the volumetric properties of aqueous 1,3-dimethyl-2-imidazolidinone between (278.15 and 318.15) K. The Journal of Chemical Thermodynamics, 41, 1424–1431. DOI: 10.1016/j.jct.2009.06.019.
Ivanov, E. V., Lebedeva, E. Yu., & Abrosimov, V. K. (2011). Volume-related interaction parameters for dilute solutions of 1,3-dimethylpropyleneurea in normal and heavy water between 278.15 K and 318.15 K. Thermochimica Acta, 513, 26–32. DOI: 10.1016/j.tca.2010.11.002.
Letcher, T., Łachwa, J., & Domańska, U. (2001). The excess molar volumes and enthalpies of (N-methyl-2-pyrrolidinone + an alcohol) at T = 298.15 K and the application of the ERAS theory. The Journal of Chemical Thermodynamics, 33, 1169–1179. DOI: 10.1006/jcht.2001.0834.
Li, J. G., Hu, Y. F., Sun, S. F., Liu, Y. S., & Liu, Z. C. (2010). Densities and dynamic viscosities of the binary system (water + 1-hexyl-3-methylimidazolium bromide) at different temperatures. The Journal of Chemical Thermodynamics, 42, 904–908. DOI: 10.1016/j.jct.2010.03.002.
Li, W. J., Zhang, Z. F., Han, B. X., Hu, S. Q., Xie, Y., & Yang, G. Y. (2007). Effect of water and organic solvents on the ionic dissociation of ionic liquids. The Journal of Physical Chemistry B, 111, 6452–6456. DOI: 10.1021/jp071051m.
Lide, D. R. (2009). CRC Handbook of chemistry and physics (89th ed). Boca Raton, FL, USA: CRC Press.
Mo, J., Liu, S. F., & Xiao, J. L. (2005). Palladium-catalyzed regioselective Heck arylation of electron-rich olefins in a molecular solvent-ionic liquid cocktail. Tetrahedron, 61, 9902–9907. DOI: 10.1016/j.tet.2005.06.066.
Olivier-Bourbigou, H., Magna, L., & Morvan, D. (2010). Ionic liquids and catalysis: Recent progress from knowledge to applications. Applied Catalysis A: General, 373, 1–56. DOI: 10.1016/j.apcata.2009.10.008.
Pereiro, A. B., Araújo, J. M. M., Oliveira, F. S., Esperança, J. M. S. S., Canongia Lopes, J. N., Marrucho, I. M., & Rebelo, L. P. N. (2012). Solubility of inorganic salts in pure ionic liquids. The Journal of Chemical Thermodynamics, 55, 29–36. DOI: 10.1016/j.jct.2012.06.007.
Petkovic, M., Seddon, K. R., Rebelo, L. P. N., & Silva Pereira, C. (2011). Ionic liquids: a pathway to environmental acceptability. Chemical Society Reviews, 40, 1383–1403. DOI: 10.1039/c004968a.
Plechkova, N. V., & Seddon, K. R. (2008). Applications of ionic liquids in the chemical industry. Chemical Society Reviews, 37, 123–150. DOI: 10.1039/b006677j.
Rey-Castro, C., & Vega, L. F. (2006). Transport properties of the ionic liquid 1-ethyl-3-methylimidazolium chloride from equilibrium molecular dynamics simulation. The effect of temperature. The Journal of Physical Chemistry B, 110, 14426–14435. DOI: 10.1021/jp062885s.
Rosenfarb, J., Huffman, H. L., Jr., & Caruso, J. A. (1976). Dielectric constants, viscosities, and related physical properties of several substituted liquid ureas at various temperatures. Journal of Chemical & Engineering Data, 21, 150–153. DOI: 10.1021/je60069a034.
Ruiz, V., Huynh, T., Sivakkumar, S. R., & Pandolfo, A. G. (2012). Ionic liquid-solvent mixtures as supercapacitor electrolytes for extreme temperature operation. RSC Advances, 2, 5591–5598. DOI: 10.1039/c2ra20177a.
Swatloski, R. P., Spear, S. K., Holbrey, J. D., & Rogers, R. D. (2002). Dissolution of cellose with ionic liquids. Journal of the American Chemical Society, 124, 4974–4975. DOI: 10.1021/ja025790m.
Tomé, L. I. N., Jorge, M., Gomes, J. R. B., & Coutinho, J. A. P. (2012). Molecular dynamics simulation studies of the interactions between ionic liquids and amino acids in aqueous solution. The Journal of Physical Chemistry B, 116, 1831–1842. DOI: 10.1021/jp209625e.
Wang, Y. T., & Voth, G. A. (2005). Unique spatial heterogeneity in ionic liquids. Journal of the American Chemical Society, 127, 12192–12193. DOI: 10.1021/ja053796g.
Yan, R. Y., Li, Z. X., Diao, Y. Y., Fu, C., Wang, H., Li, C. S., Chen, Q., Zhang, X. P., & Zhang, S. J. (2011). Green process for methacrolein separation with ionic liquids in the production of methyl methacrylate. AIChE Journal, 57, 2388–2396. DOI: 10.1002/aic.12449.
Yan, X. J., Li, S. N., Zhai, Q. G., Jiang, Y. C., & Hu, M. C. (2014). Physicochemical properties for the binary systems of ionic liquids [C n mim]Cl + N,N-dimethylformamide. Journal of Chemical & Engineering Data, 59, 1411–1422. DOI: 10.1021/je4009238.
Yang, Q. W., Xing, H. B., Cao, Y. F., Su, B. G., Yang, Y. W., & Ren, Q. L. (2009). Selective separation of tocopherol homologues by liquid-liquid extraction using ionic liquids. Industrial & Engineering Chemistry Research, 48, 6417–6422. DOI: 10.1021/ie801847e.
Yang, Q. W., Yu, K., Xing, H. B., Su, B. G., Bao, Z. B., Yang, Y. W., & Ren, Q. L. (2013). The effect of molecular solvents on the viscosity, conductivity and ionicity of mixtures containing chloride anion-based ionic liquid. Journal of Industrial and Engineering Chemistry, 19, 1708–1714. DOI: 10.1016/j.jiec.2013.02.010.
Zhang, X. P., Zhang, X. C., Dong, H. F., Zhao, Z. J., Zhang, S. J., & Huang, Y. (2012). Carbon capture with ionic liquids: overview and progress. Energy & Environmental Science, 5, 6668–6681. DOI: 10.1039/c2ee21152a.
Zhou, Q., Song, Y. T., Yu, Y. H., He, H. Y., & Zhang, S. J. (2009). Density and excess molar volume for binary mixtures of naphthenic acid ionic liquids and ethanol. Journal of Chemical & Engineering Data, 55, 1105–1108. DOI: 10.1021/je900544m.
Živković, N. V., Šerbanović, S. S., Kijevčanin, M. Lj., & Živković, E. M. (2013). Volumetric and viscometric behavior of binary systems 2-butanol + PEG 200, + PEG 400, + tetraethylene glycol dimethyl ether, and + N-methyl-2-pyrrolidone. Journal of Chemical & Engineering Data, 58, 3332–3341. DOI: 10.1021/je400486p.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bai, L., Li, SN., Zhai, QG. et al. Density, refractive index, and viscosity of binary systems composed of ionic liquids ([C n mim]Cl, n = 2, 4) and three dipolar aprotic solvents at T = 288.15–318.15 K. Chem. Pap. 69, 1378–1388 (2015). https://doi.org/10.1515/chempap-2015-0139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0139