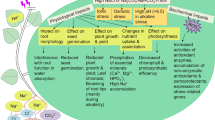
Abstract
Salt tolerance is largely dependent on a plant’ ability to maintain optimal water status in leaves. The adjustment of water relations under salinity involves changes in the transcriptional activity of genes encoding plasma membrane aquaporins (PIPs). Here, we report the effects of long-term or short-term treatments with moderate or strong salt stress on the expression of BvPIP1;1, BvPIP2;1 and BvPIP2;2 in the leaves of sugar beet, Beta vulgaris cv. Huzar, and its halophyte relative, Beta vulgaris ssp. maritima. Plants subjected to long-term treatment were watered with salt-supplemented media during a 32 day long culture period. Short-term salt treatments were executed either by immersing the petioles of excised leaves into salt solutions for 48h, or incubating excised leaf blades in salt-supplemented media for 20h. B. vulgaris ssp. maritima reacted to long-term salt treatment with a decrease in BvPIP1;1, BvPIP2;l and BvPIP2;2 expression. Contrastingly, only BvPIP2;2 transcript was down-regulated by salinity in leaves of B. vulgaris cv. Huzar, whereas BvPIP1;1 and BvPIP2;1 did not vary in response to salt-treatments. On the other hand, the expression of BvPIP1;1, BvPIP2;1 and BvPIP2;2 was enhanced by salinity if salt solutions was supplied through leaf petioles, irrespective of genotype. PIP expression in excised leaf blades revealed a complex pattern of changes. BvPIP1;1 and BvPIP2;1 expression underwent a period of transient increase in both the control and salt-treated leaves. Furthermore, BvPIP1;1 expression was enhanced by strong salinity. BvPIP2;2 expression was up-regulated by strong salinity or up- or down-regulated by moderate salinity during the treatment period.
Similar content being viewed by others
References
Aroca R. Porcel R. & Ruiz-Lozano J.M. 2011. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 63: 43–57.
Ayadi M., Cavez D., Miled N., Chaumont F. & Masmoudi K. 2011. Identification and characterization of two plasma membrane aquaporins in durum wheat (Triticum turgidum L. subsp. durum) and their role in abiotic stress tolerance. Plant Physiol. Biochem. 49: 1029–1039.
Bae E.K., Lee H., Lee J.S. & Noh E.W. 2011. Drought, salt and wounding stress induce the expression of the plasma membrane intrinsic protein 1 gene in poplar (Populus alba X P. tremula var. glandulosa). Gene 483: 43–48.
Bagatta M., Pacifico D. & Mandolino G. 2008. Evaluation of the osmotic adjustment response within the genus Beta. J. Sugar Beet Res. 45: 119–133.
Bellati J., Alleva K., Soto G., Vitali V., Jozefkowicz C. & Amodeo G. 2010. Intracellular pH sensing is altered by plasma membrane PIP aquaporin co-expression. Plant Mol. Biol. 74: 105–118.
Boursiac Y., Chen S., Luu D.T., Sorieul M., Dries N. & Maurel C. 2005. Early effects of salinity on water transport in Ara-bidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 139: 790–805.
Campos P.S., Quartin V., Ramalho J.C. & Nunes M.A. 2003. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 160: 283–292.
Chaouachi M., Alaya A., Ali I.B.H., Hafsa A.B., Nabi N., Bérard A., Romaniuk M., Skhiri F. & Said K. 2013. Development of real-time PCR method for the detection and the quantification of new endogenous references gene in sugar beet “Beta vulgaris L.”: GMO application Plant Cell. Rep. 32: 117–128.
Dadkhah A. & Moghtader S.H. 2008. Growth and gas exchange response of sugar beet (Beta vulgaris L.) cultivars grown under salt stress, pp. 1431–1434. In: Allen J.F., Gantt E., Golbeck J.H. & Osmond B. (eds), Photosynthesis. Energy from the Sun. Springer, Netherlands.
Daoud S., Harrouni C., Huchezermeyer B. & Koyro H.W. 2008. Comparison of salinity tolerance of two related subspecies of Beta vulgaris: the sea beet (Beta vulgaris ssp. maritima) and the sugar beet (Beta vulgaris ssp. vulgaris), pp. 115–129. In: Abdelly C., Öztürk M., Ashraf M. & Grignon C. (eds), Biosaline Agriculture and High Salinity Tolerance, Birkhäuser Verlag/Switzerland.
Dunajska-Ordak K., Skorupa-Kiaput M., Kurnik K., Tretyn A. & Tyburski J. 2014. Cloning and expression analysis of a gene encoding for ascorbate peroxidase and responsive to salt stress in beet (Beta vulgaris). Plant Mol. Biol. Rep. 32: 162–175.
Farkhondeh R., Nabizadeh E. & Jalilnezhad N. 2012. Effect of salinity stress on proline content, membrane stability and water relations in two sugar beet cultivars. Int. J. AgriSci. 2: 385–392.
Ghoulam C. & Fares K. 2001. Effect of salinity on seed germination and early seedling growth of sugar beet (Beta vulgaris L.). Seed Sci. Technol. 29: 357–364.
Ghoulam C., Foursy A. & Fares K. 2002. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Env. Exp. Bot. 47: 39–50.
Hachez C., Heinen R.B., Draye X. & Chaumont F. 2008. The expression pattern of plasma membrane aquaporins in maize leaf highlights their role in hydraulic regulation. Plant Mol. Biol. 68: 337–353.
Hachez C., Zelazny E. & Chaumont F. 2006. Modulating the expression of aquaporin genes in planta: A key to understand their physiological functions? Biochim. Biophys. Acta 1758: 1142–1156.
Hasanuzzaman M., Nahar K. & Fujita M. 2013. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages, pp. 25–87. In: Ahmad P. et al. (eds), Ecophysiology and Responses of Plants under Salt Stress, Springer, New York.
Heinen R.B., Ye Q. & Chaumont F. 2009. Role of aquaporins in leaf physiology. J. Exp. Bot. 11: 2971–2985.
Hoffmann C.M. 2010. Sucrose accumulation in sugar beet under drought stress. J. Agronomy Crop Sci. 196: 243–252.
Horie T., Kaneko T., Sugimoto G., Sasano S., Panda S.K., Shibasaka M. & Katsuhara M. 2011. Mechanism of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in Barley roots. Plant Cell Physiol. 52: 663–675.
Jang J.Y., Kim D.G., Kim Y.O., Kim J.S. & Kang H. 2004. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stress in Arabidopsis thaliana. Plant Mol. Biol. 54: 713–725.
Jozefkowicz C., Rosi P., Sigaut L., Soto G., Pietrasanta L.I., Amodeo G. & Alleva K. 2013. Loop A is critical for the functional interaction of two Beta vulgaris PIP aquaporins. PLOS 8: 1–13.
Katerij N., Hoorn J.W., Hamdy A., Mas-Trorilli M. & Karzel E.M. 1997. Osmotic adjustment of sugar beets in response to salinity and its influence on stomatal conductance, growth and yield. Agr. Water Manage. 34: 57–69.
Kerstiens G., Tych W., Robinson M.F. & Mansfield T.A. 2002. Sodium-related partial stomatal closure and salt tolerance of Aster tripolium. New Phytol. 153: 509–515.
Koyro H.W., Daoud S., Harrouni C. & Huchzermeyer B. 2006. Strategies of a potential cash crop halophyte (Beta vulgaris ssp. maritima) to avoid salt injury. Tropical Ecol. 47: 191–200.
Martinez-Ballesta M.C., Silva C., López-Berenguer C., Cabanero F.J., Cabañero F.J. & Carvajal M. 2006. Plant Aquaporins: New perspectives on water and nutrient uptake in saline environment. Plant Biol. 8: 535–546.
Maurel C., Verdoucq L., Luu D.T. & Santoni V. 2008. Plant aquaporins: membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 59: 595–624.
McCrea K.J. & Richardson S.G. 1987. Stomatal closure vs. osmotic adjustment: A comparison of stress responses. Crop Sci. 27: 539–543.
Mostafavi K. 2012. Effect of salt stress on germination and early seedling growth stage of sugar beet cultivars. Amer.-Eurasian J. Sust. Agricult. 6: 120–125.
Munns R. 2002. Comparative physiology of salt and water stress. Plant Cell Env. 25: 239–250.
Munns R., James R.A. & Lauchli A. 2006. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 57: 1025–1043.
Munns R. & Tester M. 2008. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59: 651–681.
Muries B., Carvajal M. & Martinez-Ballesta M.C. 2013. Response of three broccoli cultivars to salt stress, in relation to water status and expression of two leafaquaporins. Planta 235: 1298–1310.
Muries B., Mohamed F., Carvajal M. & Martinez-Ballesta M.C. 2011. Differential aquaporin expression induced by salinity in broccoli plants. Mol. Biol. Syst. 7: 1322–1335.
Liu C., Li C., Liang D., Wei Z., Zhou S., Wang R. & Ma F. 2012. Differential expression of ion transporters and aqua porins in leaves may contribute to different salt tolerance in Malus species. Plant Physiol. Bioch. 58: 159–165.
Ouziad F., Wilde P., Schmelzer E., Hildebrandt U. & Bothe H. 2006. Analysis of expression of aquaporins and Na+ /H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environ. Bot. 57: 177–186.
Pfaffl M.W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: 2002–2007.
Pfaffl M.W., Tichopad A., Prgomet C. & Neuvians T.P. 2004. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Exel-based tool using pair-wise correlations. Biotechnol. Lett. 26: 509–515.
Pakniyat H. & Armion M. 2007. Sodium and proline accumulation as osmoregulators in tolerance of sugar beet genotypes to salinity. Pak. J. Biol. Sci. 22: 4081–4086.
Prado K. & Maurel C. 2013. Regulation of leaf hydraulics: from molecular to whole plant levels. Front. Plant Sci. 4: 1–14.
Romero-Aranda R., Soria T. & Cuartero S. 2001. Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Sci. 160: 265–272.
Russell B.L., Rathinasabapathi B. & Hanson A.D. 1998. Osmotic stress induces expression of choline monooxygenase in sugar beet and amaranth. Plant Physiol. 116: 859–865.
Smethurst C.F., Gill W.M. & Shabala S. 2009. Using excised leaves to screen lucerne for salt tolerance. Physiological and cytological evidence. Plant Signal. Behav. 4: 39–41.
Steudle E. 2000. Water uptake by roots: effects of water deficit. J. Exp. Bot. 51: 1531–1542.
Suga S., Komatsu S. & Maeshima M. 2002. Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiol. 43: 1237–1237.
Uno Y., Urao T., Yamaguchi-Shinozaki K., Kanechi M., Inagaki N., Maekawa S. & Shinozaki M. 1998. Early salt-stress effects on expression of genes for aquaporin homologues in the halophyte sea aster. J. Plant Res. 111: 411–419.
Venkatesh J., Yu J.W. & Park S.W. 2013. Genomewide analysis and expression profiling of the Solarium tuberosum aquaporins. Plant Physiol. Biochem. 73: 392–404.
Very A.A., Robinson M.F., Mansfield T.A. & Sanders D. 1998. Guard cell cation channels are involved in Na+ -induced stomatal closure in a halophyte. Plant J. 14: 509–521.
Vysotskaya L., Hedley P.E., Sharipova G., Veselov D., Kudoyarova G., Morris J. & Jones H.G. 2010. Effect of salinity on water relations of wild barley plants differing in salt tolerance. AoB PLANTS. doi:10.1093/aobpla/plq006
Zhu C., Schraut D., Hartung W. & Schaffner A.R. 2005. Differential responses of maize MIP genes to salt stress and ABA. J. Exp. Bot. 56: 2971–2981.
Author information
Authors and Affiliations
Corresponding author
Supplementary data
11756_2015_7004467_MOESM1_ESM.pdf
The expression patterns of plasma membrane aquaporins in leaves of sugar beet and its halophyte relative, Beta vulgaris ssp. maritima, in response to salt stress
Rights and permissions
About this article
Cite this article
Skorupa-Kłaput, M., Szczepanek, J., Kurnik, K. et al. The expression patterns of plasma membrane aquaporins in leaves of sugar beet and its halophyte relative, Beta vulgaris ssp. maritima, in response to salt stress. Biologia 70, 467–477 (2015). https://doi.org/10.1515/biolog-2015-0056
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/biolog-2015-0056