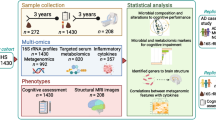
Abstract
Background
With differences apparent in the gut microbiome in mild cognitive impairment (MCI) and dementia, and risk factors of dementia linked to alterations of the gut microbiome, the question remains if gut microbiome characteristics may mediate associations of education with MCI.
Objectives
We sought to examine potential mediation of the association of education and MCI by gut microbiome diversity or composition.
Design
Cross-sectional study.
Setting
Luxembourg, the Greater Region (surrounding areas in Belgium, France, Germany).
Participants
Control participants of the Luxembourg Parkinson’s Study.
Measurements
Gut microbiome composition, ascertained with 16S rRNA gene amplicon sequencing. Differential abundance, assessed across education groups (0–10, 11–16, 16+ years of education). Alpha diversity (Chao1, Shannon and inverse Simpson indices). Mediation analysis with effect decomposition was conducted with education as exposure, MCI as outcome and gut microbiome metrics as mediators.
Results
After exclusion of participants below 50, or with missing data, n=258 participants (n=58 MCI) were included (M [SD] Age=64.6 [8.3] years). Higher education (16+ years) was associated with MCI (Odds ratio natural direct effect=0.35 [95% CI 0.15–0.81]. Streptococcus and Lachnospiraceae-UCG-001 genera were more abundant in higher education.
Conclusions
Education is associated with gut microbiome composition and MCI risk without clear evidence for mediation. However, our results suggest signatures of the gut microbiome that have been identified previously in AD and MCI to be reflected in lower education and suggest education as important covariate in microbiome studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modifiable social and behavioral risk factors of Alzheimer’s disease (AD) and related dementias convey potential to delay or prevent a substantial rate of cases, if targeted effectively (1). This entails interventions to be delivered early in the disease trajectory, informed by knowledge on working mechanisms. With respect to timeliness, research on biomarkers suggests AD-related pre-clinical pathophysiological changes occurring as early as midlife (2). At a later stage, mild cognitive impairment (MCI) reflects early, subtle changes in thinking and memory (3). While potentially due to a variety of underlying diseases or disorders, MCI is a markedly strong risk factor for AD. Furthermore, synergies in risk factors of MCI and AD exist, i.e., related to education and consequentially lifestyle (3).
Education itself reflects a well-established early-life risk factor for AD. As such, higher education is associated to lower dementia risk in later life (1). Lower dementia risk may result from education increasing cognitive abilities in early adulthood and consequent build-up of cognitive reserve, brain reserve or brain maintenance, protecting against neurodegeneration (1, 4). Moreover, risk factors such as obesity or smoking frequently cooccur, and vary in prevalence according to socioeconomic status (SES) (3, 5). Higher exposure to lifestyle-related risk factors according to education, an indicator of SES, may further contribute to a vascular pathway linking education to dementia risk. To date, there is no consensus about working mechanisms. However, recent studies suggest education, which influences life histories and in part constitutes SES, to be associated with differences in microbial community types across multiple body sites, which may be in turn associated with MCI risk (6–9).
The gut microbiome refers to a collection of microbes within the gastrointestinal tract (GIT). The GIT, reflecting the largest ecosystem of the human body, is composed of bacteria, archaea, eukaryotes, and other microbes. Composition of the gut microbiome, for instance differential abundance of specific taxa, is subject to interindividual variation, e.g., across the life-course or geographical locations (10). Factors affecting the microbiome over a lifetime are for instance linked to SES and early childhood conditions (e.g., mode of delivery, breast feeding) resulting in variation in consequent gut colonization and microbiome maturation, which may in turn continue to affect microbiome composition in later life (10-14).
Gut microbiome alterations have been observed, e.g., associated with ageing, or health. As such, ageing-related changes may result from the ageing processes (changing hormonal levels), changing health conditions (associated use of medication) or age-related behavioral changes (dietary deficiency) (10). Moreover, recent findings suggest a link of the gut microbiome to MCI or AD, and lifestyle-related risk factors such as diet or physical activity (7, 15–17). Potential working mechanisms along the gut-brain-axis likely involve complex pathways, e.g. triggering low-grade systemic inflammation by altering gut permeability or by synthesis of metabolites with neuroendocrine functions (18). Due to the likely involvement of specific molecules, the low resolution of marker gene-based microbiome analyses precludes further specification of molecular pathways.
The association of education to gut microbiome alterations and MCI risk motivate the investigation of the role of the gut microbiome in the relationship of education and MCI. Thus, we sought to examine potential mediation of the association of education and MCI by the gut microbiome in the present study.
Methods
Study Participants and Design
We analyzed data of participants, specifically the control subjects, from the Luxembourg Parkinson’s Study (LUXPARK) of the National Centre of Excellence in Research on Parkinson’s disease (NCER-PD), which received approval from the National Ethics Board (CNER Ref: 201407/13) and Data Protection Committee (CNPD Ref: 446/2017) and was conducted according to the Declaration of Helsinki (19). Eligibility criteria for analysis were age above 50, absence of Parkinson’s disease, celiac disease, and chronic inflammatory bowel disease, availability of stool samples and non-missing data. All participants provided written informed consent.
16S rRNA Gene Amplicon Sequencing Analysis
NCER-PD participants collected stool samples at home and sent them to the Integrated Biobank of Luxembourg (20). Sampling, processing, and sequencing of NCER-PD LUXPARK stool samples were done as previously described (20, 21). The 16S rRNA gene amplicon sequencing data was processed using the dadasnake workflow, a Snakemake pipeline to process amplicon sequencing data, based on DADA2 (22–24). Amplification primers were removed using cutadapt, allowing 20% mismatches and no indels (25). Quality filtering, amplicon sequence variant (ASV) generation and chimera removal were performed in DADA2. Reads were truncated at positions with less than 10 Phred score quality, or at 240 bp. The quality filtering kept only sequences with a maximum expected error of 2 and 240 bp length. Downsampling was performed to 25,000 reads using seqtk (https://github.com/lh3/seqtk:RRID:SCR_018927) and samples with smaller library sizes were removed from the downstream analysis. ASVs were generated in pooled mode for the whole study using DADA2 default parameters. For merging forward and reverse ASVs, a minimum overlap of 12 bp was required. Chimeric sequences were removed based on the consensus algorithm. Taxonomic classification was performed against SILVA v138 using the naïve Bayesian classifier implemented in mothur (26, 27). NCER-PD clinical and 16S rRNA gene amplicon sequencing data are available on request from https://www.parkinson.lu/research-participation.
Main Exposures and Outcomes
Clinical assessments were conducted by neurologists, neuropsychologists, or trained study nurses. MCI classification was based on the Montreal Cognitive Assessment (MoCA), a brief measure for assessing cognitive function (28). MoCA scores below 26 led to MCI classification (28).
Education was assessed in years (YEDU). For analysis, YEDU were grouped (0–10 [reference], 10–16, 16+ YEDu) based on the ISCED classification scheme, group sizes, and differences in compulsory schooling duration in Luxembourg for participants of different age (29, 30).
Alpha diversity captures the diversity of the microbiome within individuals. Alpha diversity will be greater in individuals with a greater number of different taxa (= richness) and/or similar abundances of prevalent taxa (= evenness). Alpha diversity is subject to variation over the life-course and higher alpha diversity has been related to better health in older age (10, 16). Three measures for alpha diversity were computed after rarefication: Chao1, Shannon and inverse Simpson (Supplementary Material). Beta diversity reflects differences of the microbiome between individuals. In that, dissimilarity indices reflect pairwise distances between individuals based on taxa abundance. In a sample-by-sample distance matrix, a greater value in a given cell indicates a larger dissimilarity between two individuals. This information can be used to compare similarity of variance and composition of the gut microbiome between groups of individuals. Two measures for beta diversity were computed: Bray-Curtis dissimilarity and Jaccard distance (Supplementary Material).
Covariates
Additional measures included sociodemographic indicators age, sex/gender, first language (French/ Luxembourgish/German versus other), partnership status (PS; married/domestic partnership versus widowed/never married/divorced/separated), body mass index (BMI), mild depressive symptoms based on the Beck Depression Inventory I (BDI-I; >9), use of antibiotic medication in the last 6 months (ATB; yes versus no), and apolipoprotein ε4 status (APOE; at least one versus no ε4 allele) (31).
Statistical Analysis
All analyses were performed in R version 4.2.0 (Supplementary Material) (32). Analysis code is available online (https://github.com/makleelux/edu_biome_mci). Differences of descriptive characteristics in presence or absence of MCI were tested with Fisher’s Exact Test for categorical and Student’s t-Test for continuous characteristics. Differences in beta diversity were tested across education groups with betadisper [vegan] and adonis2 [vegan] with 999 permutations. In short, betadisper compares average distances, i.e. the dispersion or homogeneity, across groups, while adonis2 tests multivariate differences in microbiome compositions (33).
Differential abundance analysis (DAA) was conducted across education groups, adjusting for age, sex/gender, BMI, and ATB. DAA was repeated additionally adjusting for first language, PS, BDI-I, and APOE, as robustness check. Two commonly used functions were employed (ancombc [ANCOMBC]; DESeq [DESeq 2: RRID:SCR_000154]) (34, 35). Both methods identify differentially abundant taxa with estimates of statistical significance adjusted for false discovery rates (Supplementary Material). For DAA, taxa with nonzero counts in less than 25% of samples were not tested.
Mediation analysis was specified with MCI as outcome and groups of education as exposure, adjusting for age, sex/gender, first language, PS, BDI-I, APOE and ATB. For alpha diversity as mediator, a regression-based, counterfactual approach to mediation was employed for which continuous mediator models (i.e., alpha diversity as outcome) and a logistic outcome model (i.e., MCI as outcome), including interaction terms for education and alpha diversity, were specified (Supplementary Material; cmest [CMAverse]) (36). Total effects of education on MCI were decomposed into a controlled direct effect (CDE) for alpha diversity fixed at the sample mean, a natural direct effect (NDE, Supplementary Material), and a natural indirect effect (NIE, Supplementary Material) (37–39). Proportion eliminated (PE) was calculated, indicating the proportion of the effect due to either mediation, interaction, or both, that would be eliminated by fixing the mediator to a specific level, i.e., the sample mean of the z-standardized alpha diversity measures (38). As a sensitivity check, mediation analysis was repeated without interaction terms in the outcome model.
For beta diversity as mediator, a previously described inverse-regression-based approach to mediation was employed at genus level (40, 41). In short, this approach specifies regressions for potentially mediating taxa at genus level on education, and MCI adjusted for education, in turn utilizing resulting p values to test mediation. Two functions were used, allowing to estimate mediation by abundance of specific taxa or by the overall composition of the microbiome (ldm [LDM]; permanovaFL [LDM]), while controlling for false discovery rates (40–42). Ldm suggests mediation if education affects the microbiome and consequentially the outcome. This can be tested globally (community contains any mediating taxa) and locally (mediation by specific taxa). PermanovaFL is a distance-based procedure, and suggests mediation if education affects some part of the community and some potentially different part of the community proceeds to affect MCI, thus being less conservative. For ldm an omnibus test was conducted combining analysis at three scales (i.e., relative abundance, arcsin-root transformed relative-abundance, presence-absence) (43). For permanovaFL individual and omnibus tests were conducted combining analysis at two scales (i.e., relative abundance, presence-absence) (43).
Results
Participant Characteristics
From n=524 participants of the LUXPARK study without Parkinson’s disease or Parkinsonism diagnosis, n=258 participants were eligible for analysis (M [SD] Age=64.6 [8.3] years) after exclusion of participants below age 50 (n=93), with celiac disease (n=6) or chronic inflammatory bowel disease (n=5), missing data (n=11) or without stool samples and microbiome data (n=149, and n=2 after pruning of samples with library size <10,000). Participants with MCI (n=58) were older, more likely male, had fewer YEDU and a higher BMI (Table 1). A total of 1,150 taxa at seven taxonomic ranks were identified after trimming of ASVs occurring in <10% of samples and pruning of samples with library size <10,000.
Alpha diversity as per Chao1 was lower in but not significantly associated with MCI (Table 1). Education groups did not differ significantly in beta dispersion, tested with anova (p=.17), thus meeting the assumption of homogeneity of variances for adonis2. Education groups did not differ significantly regarding multivariate analyses with adonis2 (p=.20; adjusting for sex/gender, age, ATB, BDI-I, first language, PS, and APOE), suggesting similar composition of the microbiome. However, alpha diversity was lower in lower education (Supplementary Figure 1) and was significantly lower in older age but only in lower education (Figure 1).
Beta diversity differed significantly across education groups (betadisper: p=.048; adonis2: p=.04; Figure 2), when restricting to age 65 and older.
There were no significant differences in beta diversity between MCI or age groups (Supplementary Figure 2). As Chao1 likely reflects an underestimate of richness with ASVs based on DADA2, analyses were repeated with observed richness as measure of alpha diversity. These analyses yielded analogous findings (results not shown).
Differential Abundance Analysis
DAA suggested higher relative abundance of Bacilli (class), Actinobacteria (class), Lactobacillales (order), Streptococcaceae (family), Streptococcus (genus), with DESeq 2 and Lachnospiraceae UCG 001 (genus) and two ASVs with ancombc in higher compared to lower (0–10 YEDU) education, adjusting for age, sex/gender, BMI, and ATB (Table 2).
Sensitivity analysis with additional adjustments for BDI-I, first language, PS, and/or APOE replicated findings, except for Actinobacteria and Streptococcus with DESeq 2, and the two ASVs with ancombc. There was no overlap between DESeq 2 and ancombc (of note, padj=0.07 for Lachnospiraceae UCG 001 with DESeq 2). Visual inspection of relative abundance plots suggests dose-response relationships of increasing YEDU and increasing relative abundance Lachnospiraceae UCG 001 (Supplementary Figure 3).
Mediation by Alpha Diversity
With 0–10 YEDU as reference, higher education was associated with higher Chao1 (11–16 years=0.42 [95% CI 0.07, 0.77]; 16+ years=0.38 [95% CI 0.00, 0.76]; Supplementary Table 1). With an interaction term for education and Chao1 in the outcome model, higher education was associated to lower likelihood of MCI (11–16 years=−1.24 [95% CI −2.12, −0.35]; 16+ years=−1.26 [95% CI −2.22, −0.30]) whereas greater Chao1 was not significantly associated with MCI (coefficient=−0.14, [95% CI −0.76, 0.44]). Interaction terms were not significant (11–16 YEDU:Chao1=−0.02 [95% CI −0.80, 0.79]; 16+ YEDU:Chao1=−0.20 [95% CI −1.00, 0.62]). With Chao1 as mediator, NDE (16+ YEDU) was 0.35 ([95% CI 0.15, 0.81], p=.02, Table 3) and NIE (16+ YEDU) was 0.89 ([95% CI 0.68, 1.14], p=.33) suggesting an association of education to lower MCI risk, not mediated by Chao1 (total effect=0.31 [95% CI 0.14, 0.72], p=.008; CDE=0.33 [95% CI 0.14, 0.78], p=.02). The proportion eliminated, PE=0.22 ([95% CI 0.00, 0.59], p=.049), suggests most of the association of education on MCI risk being due to a direct effect of education but also a significant amount due to interaction, mediation, or both.
Given the moderately rare outcome (∼22.5% MCI), CDE, NDE and NIE reported on the odds-ratio scale may be overestimated. As a sensitivity analysis, estimation was repeated on the risk-ratio scale using a multinomial log-linear link for the outcome model, resulting in a similar pattern of findings but without significant PE (results not shown) (37).
Analyses without interaction terms led to similar result patterns in regression models (Supplementary Table 1). Comparison of 0–10 to 11–16 YEDU led to similar result patterns in effect decomposition (Table 3). BMI was hypothesized as a potential mediator of education and MCI, or microbiome diversity and MCI, and thus not included in the main analyses but considered for robustness checks. Inclusion of BMI led to attenuated associations of education with Chao1 in the mediator, and of Chao1 with MCI in the outcome model. This in turn led to attenuated NIE and a similar, but no longer significant estimate of PE (results not shown).
Analyses with Shannon or inverse Simpson as mediator suggested similar findings but no significant PE. Analyses with inverse Simpson as mediator suggested similar findings except for no significant association of education with alpha diversity in the mediator model and a significant proportion of the total effect of education due to (additive) interaction, when comparing 0–10 to 11–16 YEDU (regression models: Supplementary Tables 2, 3, effect decomposition: Supplementary Tables 4, 5).
Mediation by Beta Diversity
Ldm suggested no significant mediation by individual taxa or by the composition of the microbiome (p=.99 for n=48,000 completed permutations with ldm.omni3). Likewise, permanovaFL suggested no significant mediation by the composition of the microbiome, on the relative abundance (Bray-Curtis dissimilarity, p=.70), or presence-absence scale (Jaccard dissimilarity, p=.35), or overall (p=.54 for n=600 completed permutations with permanovaFL.omni). Robustness checks (with BMI) yielded a similar pattern of findings (results not shown).
Discussion
Higher education was associated with a lower risk of MCI, with most of this association not being due to mediation by the gut microbiome. Despite differences in taxonomic signatures and gut microbiome composition between education groups, our findings suggest no significant mediation of the association of education with MCI by measures of alpha diversity or individual taxa. However, effect decomposition indicated potential additive interaction between education and alpha diversity.
General Discussion
In this study, MCI risk was highest in the group with 0–10 YEDU. Higher education groups did not differ in their association with MCI. This reflects earlier findings suggesting that education is related to reserve capacity, and thus lower MCI risk, by in particular increasing levels of cognitive skills in early life which then persist until old age (4).
Critically, more than 16 YEDU likely reflect education beyond the end of adolescence, with positive effects levelling off and thus, no linear association of education with MCI.
Further analyses suggested a dominating direct effect of education. While education was associated with microbial diversity, no indicator of diversity was significantly associated to MCI, although less clear so for Chao1, reflecting richness, in models without interaction terms. Nonetheless, one fifth of the association of education on MCI could be removed (proportion eliminated) by intervening to fix Chao1 at the sample mean. Four-way decomposition suggests this to be most likely attributable to an additive interaction of education and Chao1, such that their association with lower MCI risk increases with increments in education (38). Of note, this finding reinforces most of the association of education with MCI to be flowing through a direct causal path, which is also supported by sensitivity analysis on the risk-ratio scale.
A potential explanation for the absence of statistically significant mediation would be that lower education may proxy higher MCI risk due to factors which are not associated with the gut microbiome, such as cognitive stimulation. In that case, the observed variation in gut microbiome diversity and composition across education groups would not be causally related to MCI risk.
However, our findings highlight education-related gut microbiome diversity and composition reflecting those found in MCI and AD. Given MCI as a strong risk factor and AD as the most common cause of dementia, similarities in the gut microbiota of individuals with low education – who are at higher risk of dementia – and of people living with AD may indicate further mechanisms contributing to the disease. These may involve nutritional choices and chronic low-grade inflammation or the synthesis of metabolites leading to modulation of nerve signaling via the enteric nervous system. A previous study found reduced richness as well as a distinct composition of the gut microbiome in terms of beta diversity in participants with AD compared to healthy controls (44). In line with a hypothesized neurodegenerative pathway involving education and the gut-brain-axis, our findings suggest that lower education is associated with reduced richness and a distinct gut microbiome composition. Conversely, another study found increasing richness with AD progression, which may be explained by an apparent gradient of education from lowest (in unimpaired cognition) to highest (in moderate AD) (45). Considering our findings lower education may not only have altered the likelihood of belonging to patient or control groups but may also have resulted in different taxonomic signatures.
Previous findings suggest similar alterations with respect to reduced alpha diversity in lower income and area-level SES settings (8, 9). Education is related to income and wealth, and consequently with selection into areas with fewer socioeconomic resources. As such, education may capture community-level or spatial exposures affecting gut microbiome composition (9).
Critically, we found Chao1 to be lower in older age, but only in participants with lower education. Additionally, compositional differences by education were only significant in older age. This extends on earlier reports of interindividual variability and reduced biodiversity in later life by suggesting education as a key modifier (46). Moreover, our finding of lower alpha diversity in lower education, suggests a putative association with a dysbiotic state. While lower alpha diversity has been discussed previously as a possible indicator of AD, to date, no concrete link has been established between education and dysbiosis and consequently AD or MCI (16, 47). This may be due to the relatively limited depth and linked resolution of sequencing or the breadth of education measures (16).
Extending on mediation results with alpha diversity metrics, ldm and permanovaFL did not identify mediating taxa or compositional changes translating into decreased MCI risk (40–42). However, DAA results suggest differential abundance in line with an MCI or AD phenotype and consequentially a potential communality of lower education and AD pathology. Bacilli (class), Actinobacteria (class), Lactobacillales (order), Streptococcaceae (family), Streptococcus (genus), Lachnospiraceae UCG 001 (genus) and two ASVs were depleted in lower education.
Contrary to our findings given a hypothesized link of education to MCI via the gut, previous studies showed an increased ratio of Firmicutes to Bacteroidetes (F/B), and an increased relative abundance of Lactobacillales in AD, and of Firmicutes in MCI (17, 48). However, earlier findings were likely driven by depleted Bacteroides; increases in Firmicutes were not statistically significant (17, 48). Other studies found increased Bacteroidetes in MCI without AD and, in line with our findings, depletion of Firmicutes in AD and amnestic MCI (7, 44, 49). Increased Bacteroides may relate to impaired cognition potentially through cerebral small vessel disease and resulting white matter hyperintensities and, in line with our findings, education may alter brain reserve to such damage via increased node degree (7, 50).
Lachnospiraceae UCG 001 were earlier found to be depleted in participants with more severe depressive symptoms, implying impaired synthesis of short-chain fatty-acids, such as butyrate and other depression-related neurotransmitters (51). Recent findings further suggest lower cognitive performance due to decreased levels of butyrate following stool transplantation of sleep deprived to control mice (52). Depletion of Lachnospiraceae UCG 001 in lower education may be associated to lower cognitive performance and MCI classification in line with a phenotype related to depressive symptom severity. Since we adjusted for BDI-I, education and depressive symptom severity may share a common neuroendocrinal pathway to impaired cognition, i.e., via nutritional choices, involving Lachnospiraceae UCG 001 and metabolites synthesized by gut microbiota.
Moreover, two differentially abundant ASV were identified, one classified as Lachnospiraceae UCG 001, the other as NK4A214 group, albeit classification comes with some uncertainty (Species unidentified, Supplementary Table 6).
One study found Actinobacteria and Streptococcus enriched in mild and moderate AD, potentially explained by higher educational attainment in AD-groups compared to controls, given our findings of higher abundance of Actinobacteria and Streptococcus in higher education (45). Consequently, both depleted Actinobacteria and Streptococcus in lower education may reflect an AD phenotype but the alternative explanation that their alteration reflects educational differences cannot be ruled out. Of note, Actinobacteria was earlier found depleted in AD compared to healthy controls suggesting e.g., detriments to intestinal barrier integrity in both AD and lower education (44).
Limitations and Future Directions
In this study, we extensively triangulated potential mediation in a large cohort and point to potential interaction of education and gut microbiome diversity regarding MCI risk. Despite careful adjustment, residual confounding may bias results. Effect decomposition assumes no unmeasured (i) exposure-outcome, (ii) mediator-outcome, or (iii) exposure-mediator confounding, and (iv) that (ii)-confounders are not affected by the exposure. However, PE and CDE do not require (iii) or (iv), and NDE is robust to (iv), assuming monotone associations (53, 54). MCI classification was based on a screening instrument. Differences in causes underlying MCI classification may bias DAA, which we could not formally assess (3). Moreover, SES was not formally addressed in the present analyses and may reflect a common cause of or indirect causal path variable of educational differences and MCI risk. However, DAA was carefully adjusted for different confounder sets, including lifestyle-related variables and risk factors of impaired cognition, such as BMI, depressive symptoms, or partnership status. Grouping of education may bias estimates, although the associations of YEDU with MCI is likely non-linear. Further, compulsory schooling years vary across birthyears (29). Grouping by less than 10 YEDU selects older participants or those that immigrated. However, analysis was adjusted for age and first language as proxy for immigration. Limited diversity and sample size in this cohort prevented subgroup analysis and hampers generalizability (Supplementary Table 7). Further analysis regarding functional categories and diversity are necessary to fully elucidate implications of distinct taxonomic signatures (55).
Conclusion
Our results suggest signatures of the gut microbiome that have been identified previously in AD and MCI to be reflected in lower education. We show that most of the association of education with MCI is of a direct nature and stress the importance of considering social determinants of health, specifically education, as key modifiers in microbiome studies. Our findings underline the potential of the gut microbiome as a biomarker and intervention target regarding MCI, which is promising, considering its modifiability until later life. Future research with longitudinal survey designs is required to further investigate potential interaction of education and the gut microbiome and their implication for neurodegenerative diseases.
Change history
10 March 2024
An Erratum to this paper has been published: https://doi.org/10.14283/jpad.2024.53
References
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet Lond Engl. 2020;396(10248):413–446. doi:https://doi.org/10.1016/S0140-6736(20)30367-6
Zetterberg H, Bendlin BB. Biomarkers for Alzheimer’s disease—preparing for a new era of disease-modifying therapies. Mol Psychiatry. 2021;26(1):296–308. doi:https://doi.org/10.1038/s41380-020-0721-9
2022 Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc. 2022;18(4):700–789. doi:https://doi.org/10.1002/alz.12638
Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM. Education and Cognitive Functioning Across the Life Span. Psychol Sci Public Interest. 2020;21(1):6–41. doi:https://doi.org/10.1177/1529100620920576
Deckers K, Cadar D, van Boxtel MPJ, Verhey FRJ, Steptoe A, Köhler S. Modifiable Risk Factors Explain Socioeconomic Inequalities in Dementia Risk: Evidence from a Population-Based Prospective Cohort Study. J Alzheimers Dis. 2019;71(2):549–557. doi:https://doi.org/10.3233/JAD-190541
Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509(7500):357–360. doi:https://doi.org/10.1038/nature13178
Saji N, Murotani K, Hisada T, et al. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9(1):19227. doi:https://doi.org/10.1038/s41598-019-55851-y
Miller GE, Engen PA, Gillevet PM, et al. Lower Neighborhood Socioeconomic Status Associated with Reduced Diversity of the Colonic Microbiota in Healthy Adults. PloS One. 2016;11(2):e0148952. doi:https://doi.org/10.1371/journal.pone.0148952
Bowyer RCE, Jackson MA, Le Roy CI, et al. Socioeconomic Status and the Gut Microbiome: A TwinsUK Cohort Study. Microorganisms. 2019;7(1):17. doi:https://doi.org/10.3390/microorganisms7010017
Greenhalgh K, Meyer KM, Aagaard KM, Wilmes P. The human gut microbiome in health: establishment and resilience of microbiota over a lifetime. Environ Microbiol. 2016;18(7):2103–2116. doi:https://doi.org/10.1111/1462-2920.13318
Wampach L, Heintz-Buschart A, Fritz JV, et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat Commun. 2018;9(1):5091. doi:https://doi.org/10.1038/s41467-018-07631-x
Milcent C, Zbiri S. Prenatal care and socioeconomic status: effect on cesarean delivery. Health Econ Rev. 2018;8(1):7. doi:https://doi.org/10.1186/s13561-018-0190-x
Heck KE, Braveman P, Cubbin C, Chávez GF, Kiely JL. Socioeconomic Status and Breastfeeding Initiation Among California Mothers. Public Health Rep. 2006;121(1):51. doi:https://doi.org/10.1177/003335490612100111
Lee SA, Lim JY, Kim BS, et al. Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr Res Pract. 2015;9(3):242–248. doi:https://doi.org/10.4162/nrp.2015.9.3.242
Alkasir R, Li J, Li X, Jin M, Zhu B. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8(2):90–102. doi:https://doi.org/10.1007/s13238-016-0338-6
Cabrera C, Vicens P, Torrente M. Modifiable Risk Factors for Dementia: The Role of Gut Microbiota. Curr Alzheimer Res. 2021;18(13):993–1009. doi:https://doi.org/10.2174/1567205018666211215152411
Saji N, Niida S, Murotani K, et al. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9(1):1008. doi:https://doi.org/10.1038/s41598-018-38218-7
Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–255. doi:https://doi.org/10.1038/s41579-020-00460-0
Hipp G, Vaillant M, Diederich NJ, et al. The Luxembourg Parkinson’s Study: A Comprehensive Approach for Stratification and Early Diagnosis. Front Aging Neurosci. 2018;10:326. doi:https://doi.org/10.3389/fnagi.2018.00326
Baldini F, Hertel J, Sandt E, et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020;18(1):62. doi:https://doi.org/10.1186/s12915-020-00775-7
Wilmes P, Trezzi JP, Aho V, et al. An archaeal compound as a driver of Parkinson’s disease pathogenesis. Published online July 26, 2022. doi:https://doi.org/10.21203/rs.3.rs-1827631/v1
Weißbecker C, Schnabel B, Heintz-Buschart A. Dadasnake, a Snakemake implementation of DADA2 to process amplicon sequencing data for microbial ecology. GigaScience. 2020;9(12):giaa135. doi:https://doi.org/10.1093/gigascience/giaa135
Mölder F, Jablonski KP, Letcher B, et al. Sustainable data analysis with Snakemake. F1000Research. 2021;10:33. doi:https://doi.org/10.12688/f1000research.29032.2
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. doi:https://doi.org/10.1038/nmeth.3869
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17(1):10–12.
Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(D1):D590–D596. doi:https://doi.org/10.1093/nar/gks1219
Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541. doi:https://doi.org/10.1128/AEM.01541-09
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:https://doi.org/10.1111/j.1532-5415.2005.53221.x
Honig, M. S., Bock T. Luxembourg–ECEC Workforce Profile. In: Oberhuemer P, Schreyer I, eds. Workforce Profiles in Systems of Early Childhood Education and Care in Europe.; 2017. http://www.seepro.eu/English/pdfs/LUXEMBOURG_Workforce.pdf
UNESCO. International Standard Classification of Education 2011. UNESCO Institute for Statistics; 2012.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An Inventory for Measuring Depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi:https://doi.org/10.1001/archpsyc.1961.01710120031004
R Core Team. R: A Language and Environment for Statistical Computing. Published online April 22, 2022. https://www.R-project.org/
Oksanen J., Simpson G. L., Blanchet F. G., et al. vegan: Community Ecology Package. R package version 2.6–2. Published online 2022.
Lin H, Peddada SD. Analysis of compositions of microbiomes with bias correction. Nat Commun. 2020;11(1):3514. doi:https://doi.org/10.1038/s41467-020-17041-7
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq 2 . Genome Biol. 2014;15(12):550. doi:https://doi.org/10.1186/s13059-014-0550-8
Shi B, Choirat C, Coull BA, VanderWeele TJ, Valeri L. CMAverse: A Suite of Functions for Reproducible Causal Mediation Analyses. Epidemiology. 2021;32(5):e20. doi:https://doi.org/10.1097/EDE.0000000000001378
Valeri L, VanderWeele TJ. Mediation analysis allowing for exposuremediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi:https://doi.org/10.1037/a0031034
VanderWeele TJ. A unification of mediation and interaction: a four-way decomposition. Epidemiol Camb Mass. 2014;25(5):749. doi:https://doi.org/10.1097/EDE.0000000000000121
VanderWeele TJ, Vansteelandt S. Mediation Analysis with Multiple Mediators. Epidemiol Methods. 2014;2(1):95–115. doi:https://doi.org/10.1515/em-2012-0010
Yue Y, Hu YJ. A new approach to testing mediation of the microbiome at both the community and individual taxon levels. Bioinforma Oxf Engl. 2022;38(12):3173–3180. doi:https://doi.org/10.1093/bioinformatics/btac310
Yue Y, Hu YJ. Extension of PERMANOVA to Testing the Mediation Effect of the Microbiome. Genes. 2022;13(6):940. doi:https://doi.org/10.3390/genes13060940
Hu YJ, Satten GA. Testing hypotheses about the microbiome using the linear decomposition model (LDM). Bioinforma Oxf Engl. 2020;36(14):4106–4115. doi:https://doi.org/10.1093/bioinformatics/btaa260
Zhu Z, Satten GA, Hu YJ. Integrative analysis of relative abundance data and presence-absence data of the microbiome using the LDM. Robinson P, ed. Bioinformatics. 2022;38 (10):2915–2917. doi:https://doi.org/10.1093/bioinformatics/btac181
Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. doi:https://doi.org/10.1038/s41598-017-13601-y
Chen L, Xu X, Wu X, et al. A comparison of the composition and functions of the oral and gut microbiotas in Alzheimer’s patients. Front Cell Infect Microbiol. 2022;12:942460. doi:https://doi.org/10.3389/fcimb.2022.942460
Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacol Res. 2013;69(1):11–20. doi:https://doi.org/10.1016/j.phrs.2012.10.005
Li Z, Zhou J, Liang H, et al. Differences in Alpha Diversity of Gut Microbiota in Neurological Diseases. Front Neurosci. 2022;16:879318. doi:https://doi.org/10.3389/fnins.2022.879318
Nagpal R, Neth BJ, Wang S, Craft S, Yadav H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019;47:529–542. doi:https://doi.org/10.1016/j.ebiom.2019.08.032
Liu P, Wu L, Peng G, et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019;80:633–643. doi:https://doi.org/10.1016/j.bbi.2019.05.008
DeJong NR, Jansen JFA, van Boxtel MPJ, et al. Cognitive resilience depends on white matter connectivity: The Maastricht Study. Alzheimers Dement. 2023;19(4):1164–1174. doi:https://doi.org/10.1002/alz.12758
Radjabzadeh D, Bosch JA, Uitterlinden AG, et al. Gut microbiome-wide association study of depressive symptoms. Nat Commun. 2022;13(1):7128. doi:https://doi.org/10.1038/s41467-022-34502-3
Wang X, Wang Z, Cao J, Dong Y, Chen Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 2023;11(1):17. doi:https://doi.org/10.1186/s40168-022-01452-3
Pearl J. Direct and indirect effects. In: Proceedings of the 17th Annual Conference on Uncertainty in Artificial Intelligence (UAI-01). Morgan Kaufmann; 2001:411–442. doi:https://ftp.cs.ucla.edu/pub/stat_ser/R273-U.pdf
Tchetgen EJT, VanderWeele TJ. On identification of natural direct effects when a confounder of the mediator is directly affected by exposure. Epidemiol Camb Mass. 2014;25(2):282–291. doi:https://doi.org/10.1097/EDE.0000000000000054
Heintz-Buschart A, Wilmes P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018;26(7):563–574. doi:https://doi.org/10.1016/j.tim.2017.11.002
Acknowledgments
Data used in the preparation of this manuscript were obtained from the National Centre of Excellence in Research on Parkinson’s Disease (NCER-PD). The National Centre of Excellence in Research on Parkinson’s Disease (NCER-PD) is funded by the Luxembourg National Research Fund (FNR/NCER13/BM/11264123). We would like to thank all participants of the Luxembourg Parkinson’s Study for their important support of our research. Furthermore, we acknowledge the joint effort of the NCER-PD Consortium members from the partner institutions Luxembourg Centre for Systems Biomedicine, Luxembourg Institute of Health, Centre Hospitalier de Luxembourg, and Laboratoire National de Santé generally contributing to the Luxembourg Parkinson’s Study as listed below.
Funding
Funding: This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme [grant number 803239, to AKL and grant agreement No. 863664 to PW]. This research was funded in part, by the Luxembourg National Research Fund (FNR), grant reference ([FNR/NCER13/BM/11264123], PEARL program [FNR/ P13/6682797 to RK], MotaSYN [12719684 to RK], MAMaSyn [to RK], MiRisk-PD [C17/BM/11676395 to RK, PM], and the FNR/German Research Foundation [DFG] Core INTER [ProtectMove, FNR11250962 to PM]). For the purpose of open access, and in fulfilment of the obligations arising from the grant agreement, the author has applied a Creative Commons Attribution 4.0 International (CC BY 4.0) license to any Author Accepted Manuscript version arising from this submission. AKL, RK and PW acknowledge financial support of the Institute for Advanced Studies of the University of Luxembourg through an AUDACITY grant (ref. no. MCI-BIOME_2019). Mr Klee and Dr Leist are funded by the European Research Council. No other financial disclosures were reported by the authors of this paper. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Author Contribution: Matthias Klee: Conceptualization, Methodology, Formal analysis, Investigation, Writing–Original Draft, Writing–Review & Editing, Visualization; Velma T. E. Aho: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing–Review & Editing, Supervision; Patrick May: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing–Review & Editing, Supervision; Anna Heintz-Buschart: Methodology, Investigation, Resources, Data Curation, Writing–Review & Editing; Zied Landoulsi: Data Curation, Writing–Review & Editing; Sonja R. Jónsdóttir: Data Curation, Writing–Review & Editing; Claire Pauly: Data Curation, Writing–Review & Editing; Lukas Pavelka: Data Curation, Writing–Review & Editing; Léa Delacour: Writing–Review & Editing, Project administration; Anne Kaysen: Writing–Review & Editing, Project administration; Rejko Krüger: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing–Review & Editing, Project administration, Funding acquisition; Paul Wilmes: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing–Review & Editing, Supervision, Project administration, Funding acquisition; Anja K. Leist: Conceptualization, Methodology, Investigation, Writing–Original Draft, Writing–Review & Editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflicts: None of the authors reports a conflict of interest related to the submission of the manuscript. Dr Leist received remuneration from Roche for advisory activities related to expanding health equity in AD. No other interest has been declared.
Ethical standards: The Luxembourg Parkinson’s Study (LUXPARK) of the National Centre of Excellence in Research on Parkinson’s disease (NCER-PD) received approval from the National Ethics Board (CNER Ref: 201407/13) and Data Protection Committee (CNPD Ref: 446/2017) and was conducted according to the Declaration of Helsinki. All participants provided written informed consent.
Additional information
Consent Statement: All participants provided written informed consent.
Supplementary Material
Rights and permissions
Open Access : This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Klee, M., Aho, V.T.E., May, P. et al. Education as Risk Factor of Mild Cognitive Impairment: The Link to the Gut Microbiome. J Prev Alzheimers Dis 11, 759–768 (2024). https://doi.org/10.14283/jpad.2024.19
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2024.19