Abstract
Background
Alzheimer’s disease (AD) is a neurodegenerative disease with complex disease etiology and pathological processes. These include formation of plaques and tangles, aberrant lipid processing, neuroinflammation, cerebrovascular dysregulation, ion channel and mitochondrial dysfunction, and oxidative stress. Disease-modifying therapies focusing on all these different facets are needed. TW001 is an oral formulation with the radical scavenger edaravone as its active ingredient, targeting oxidative stress.
Objectives
This manuscript describes the trial design for Phase IIA Alzheimer Study Using oRal Edaravone (ASURE).
Methods
ASURE is a randomized, placebo-controlled, proof-of-concept study aiming to evaluate safety and target engagement following administration of TW001 in early AD patients. Patients should have a biomarker confirmed diagnosis to be included in the trial and will be treated for 90 days. The primary endpoints include safety and effect of TW001 on oxidative stress biomarkers. Exploratory endpoints focus on a panel of AD(-related) fluid-based biomarkers and EEG. In addition, a recently developed cognitive functional composite (CFC) score will measure early signs of cognitive and functional effects of TW001.
Results
This article outlines the design of the clinical study, no results are included.
Conclusions
The ASURE trial design is discussed, with a particular focus on fluid biomarkers, EEG, and CFC as endpoints. By testing multiple measures related to pathology, pharmacodynamics, EEG as proxy for cognition, and cognitive functional scores, it is expected that small changes will be detectable in trials of shorter duration. Moreover, the wide range of endpoints allows to make well-informed decisions for designing pivotal studies later.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia affects over 50 million people worldwide, and it is expected that this number will only increase (1). Recently, a new era of drug development for AD started by the approval of monoclonal antibodies against amyloid by the Food and Drug Administration (FDA). However, we still need improved disease modifying therapies (DMTs) to treat AD.
Extracellular plaques constituted of amyloid β (Aβ) peptides and intracellular neurofibrillary tangles comprised of phosphorylated tau (pTau) are important hallmarks of AD pathology (2, 3). However, in AD many additional pathological processes are occurring, including neuroinflammation, cerebrovascular dysregulation, ion channel and mitochondrial dysfunction, and oxidative stress (4, 5). Together, these processes result in neurodegeneration and subsequently, the clinical end stage dementia. DMTs targeting many of these pathological pathways are needed, and efficacy and disease modification need to be investigated in well-designed trials.
The occurrence of oxidative stress in the brain of AD patients can be related to many of the ongoing pathological processes mentioned above. Aβ peptides can interact with metals making them highly reactive, inducing the formation of reactive oxygen species (ROS), resulting in oxidative stress (6–8). Furthermore, Aβ-induced ROS formation can cause protein and lipid oxidation resulting in protein dysfunction and membrane alterations, respectively (9). Additionally, Aβ oligomers interact with the NMDA receptor, which, through a cascade of reactions, leads to mitochondrial dysfunction and further promote oxidative stress (7, 10). ROS also interact with the tau protein, inducing hyperphosphorylation of tau (11). Lastly, the ROS affect microglia and cause activation of astrocytes and the innate immune system, creating a pro-inflammatory environment (12). Combined, these effects create a positive feedback loop, causing further disease progression (13).
Edaravone donates an electron to the free radical products, thereby ameliorating oxidative and nitrosative stress (14–18). Edaravone has been proven to be effective in in vitro AD models by attenuating Aβ-induced oxidative stress, mitochondrial dysfunction and neurotoxicity (19–21). In vivo, edaravone improved cognitive function in transgenic Alzheimer mice (APP/PS1) (22). Based on these findings and the general role of oxidative stress in AD, it is expected that edaravone has a multi-factorial effect. It is anticipated that TW001, an oral formulation of edaravone, could provide convenient at-home dosing, with the aim to halt disease progression.
To explore the effect of TW001 in AD, Treeway in collaboration with the Alzheimer Center in Amsterdam, initiated ASURE, a Phase IIA, randomized, double-blind, placebo-controlled study, to investigate the safety and effectiveness of TW001 in patients with mild to moderate AD. Although the primary objective of this innovative study is to investigate safety and determine target engagement in AD patients, the study will also explore the early effect of TW001 on a variety of biomarkers, as well as the potential clinical effects.
Methods
Design
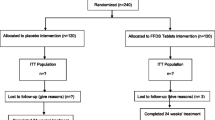
ASURE is a multi-center, randomized, placebo-controlled clinical trial. It is a proof-of-concept study aiming to evaluate the safety, pharmacodynamics, and pharmacokinetics of TW001 in patients with Alzheimer’s disease. Approximately 150 patients will be screened for eligibility for the trial in 9 sites located in Europe. The aim is to enroll approximately 60 patients, who will be randomized 1:1 to receive 100 mg of TW001 or placebo once daily for 90 days. Due to reduced bioavailability of approximately 40% when taken with food, it is recommended patients take the drug in fasted condition. Randomization will be achieved using an interactive web response system (IWRS), which also aids in maintaining double-blindness. Packaging will be done according to the national law and GMP requirements.
Study population
Both male and female patients ≥ 55 to ≤ 80 years old with a body mass index between ≥ 18.5 to ≤ 30.0 kg/m2 are included. Patients eligible to participate in the trial must have a diagnosis of MCI due to AD or mild AD dementia as defined by the NIA-AA criteria research framework (3). This includes patients with an MMSE score of ≥ 20 at screening and A+T+N+ or A+T+N- based upon: CSF profile consistent with AD taken during screening period, documented evidence of a CSF profile consistent with AD obtained within previous 12 months, or documented amyloid PET scan evidence acquired within previous 12 months. Additionally, the patient must have a study partner available who is willing to accompany the patient to all visits. If the patient is taking medication, supplements or vitamins that may have an influence on oxidative stress, cognition and/or EEG, the dose must be stable at screening for at least one month, and must remain stable for the duration of the trial.
Patients will be excluded if there is a known history of stroke, evidence of a clinically relevant neurological disorder other than AD at screening, a history of seizures or epilepsy within the last 5 years before screening, evidence of a clinically relevant unstable psychiatric disorder based on the DSM-5™ criteria, or renal impairment as indicated by a creatinine clearance of less than 50 mL/min. Patients that have a history of hepatic disease, AST or ALT levels of ≥ 2 times upper limit of normal, biliary tract disease, or a positive screening test for HIV, hepatitis B or C will be excluded. Also, there should be no evidence of unstable cardiac, pulmonary, endocrine, hematologic, or active infectious diseases or a history of cancer within 3 years prior to screening. Since a lumbar puncture is part of the trial procedures, any contraindication of obtaining a lumbar puncture is an exclusion criterion.
Study procedures
Patients suitable for inclusion will be asked for written informed consent. Thereafter, they will be screened based on the inclusion and exclusion criteria. Vital signs will be investigated, a 12-lead ECG and physical and neurological examination will be performed (Figure 1). Regular urinalysis, hematology, blood chemistry, and coagulation tests will be performed and serological findings will be investigated. In case no information regarding CSF profile or PET scan is available, a lumbar puncture will be performed at screening. When a lumbar puncture must be carried out, more fluid will be taken and will serve as the baseline measurement. This will minimize patient burden. Screening will be done as close as possible to the baseline visit to reduce temporal variation. Cognitive tests including MMSE and CDR-SB will also be done.
At baseline visit (V2; Day 1), urine and blood will be sampled for safety, pharmacokinetic and pharmacodynamic endpoints. A lumbar puncture will be performed if this was not done yet during screening and the patient will undergo electroencephalography (EEG). The cognitive-functional composite (CFC), a relatively new cognitive-functional test designed to measure neurological decline in early-stage dementia, will also be performed. It is expected that this test will be more sensitive to small cognitive changes compared to other well-known cognitive tests (23). On visit 3 (day 30) blood and urine will be sampled for endpoint measurements. This visit also includes an optional lumbar puncture.
At end of treatment (EOT) visit (V4; Day 90), blood, urine and CSF sampling will be performed. Furthermore, the patient will undergo EEG. Cognitive tests will be carried out, these tests include the MMSE, CFC, and CDR-SB. Physical and neurological exams will be completed. A follow-up visit at least 14 days after EOT will be done to evaluate if any adverse events occurred.
Results
Endpoints
Primary endpoints of the study are divided in pharmacodynamic and safety endpoints. 8-hydroxy-2′-deoxyguanosine (8-OHdG)/8-hydroxyguanosine (8-OHG) and 3-nitrotyrosine (3-NT) will be measured in CSF and plasma, uric acid will only be measured in plasma. 8-OHdG and 8-OHG are oxidative stress markers related to DNA-damage and RNA-damage, respectively (24–26). 3-NT is an indicator of protein damage due to oxidative stress (27). These biomarkers will also be measured in urine as explorative endpoints. Uric acid is an endogenously produced antioxidant, it is included in this trial as it works very similar to edaravone. It is important to further understand the effect of TW001 on endogenous anti-oxidants. As safety outcomes, adverse events will be monitored and changes in ECG, safety labs, and physical and neurological examination will be determined.
Furthermore, the effect of TW001 on AD pathology as measured by Aβ42, Aβ40, pTau isoforms, total Tau (tTau) are included as exploratory endpoints. Due to the expected multifactorial effect of edaravone and expected broad downstream changes, it is anticipated that there might also be a measurable change in inflammatory, general neurodegenerative and synaptic function markers (table 1). These will be measured in CSF and a subset will also be measured in plasma. The measurement of the synaptic function will also include EEG analysis.
As a cognitive endpoint, the newly developed CFC and the cognitive dementia rating scale (CDR) will be included. The CFC was developed by the Alzheimer Center Amsterdam and constitutes of seven cognitive tests and one functional test focusing on episodic and working memory, and functional impairment (28, 29). The cognitive part is completed by a neuropsychologist. The functional component, consisting of the Amsterdam IADL Questionnaire, is completed by a partner of the patient and has been extensively validated previously (30–32). Cognitive changes tend to be difficult to capture with conventional cognitive tests partly due to floor and ceiling effects (23). The CFC has been designed to capture changes in cognition in the early dementia stages and has been validated against other cognitive tests, demonstrating no floor and ceiling effects and better responsiveness to minor cognitive changes (23, 33).
TW001 pharmacokinetics will be determined as secondary endpoint in plasma. Pharmacokinetics include the area under the curve from time-point 0 to time of sampling, the maximum concentration and time of maximum concentration.
Statistical analysis
All data will be tabulated descriptively and groups statistics will be presented. The primary endpoint is to determine safety of TW001. To investigate this, effects on safety laboratory tests and adverse events will be described. Secondly the effect of TW001 on oxidative stress biomarkers in CSF and plasma will be investigated as part of the primary endpoints. Differences between baseline and end of treatment in these markers will be determined statistically. Population PK-PD modeling which includes the retrospective assessment of dose- and exposure response correlations will also be applied.
A broad range of biomarkers is included in this trial, which will all be analyzed as exploratory endpoints. Apart from providing insights into the different aspects of the disease, these biomarkers could be used to create a composite biomarker reflective of the combined effect of TW001 on the disease. Multivariate modelling will combine longitudinal models for the biomarker data with Cox regression models to establish the set of biomarkers resulting in the highest predictive value for rate of disease progression /disease duration. The longitudinal biomarker models will be complemented with models for the clinical parameters and combined in a single framework to establish a monitoring tool of the disease. Model reduction techniques will be applied to understand and account for correlations among biomarkers and clinical parameters. The finalized model should be a biomarker-based model that optimally characterizes the disease trajectory and be used to monitor AD disease progression and treatment effects in clinical trials as well as clinical practice. To this end, the data obtained from ASURE (including the quantified biomarker and patient-derived clinical and demographic data) will need to be subjected to a variety of statistical multivariate analyses, each of them tailored to the specific application.
Discussion
Here we describe the innovative design of the ASURE study to investigate safety, pharmacodynamics and pharmacokinetics of TW001 in AD patients. The main objective is to examine the effect of TW001 on oxidative stress related biomarkers and evaluate its safety in AD patients. Moreover, the development of a composite biomarker can give insights into disease progression.
Based on preclinical evidence, the mechanism of action of edaravone in the treatment of AD is thought to be multifactorial, comprising the mitigation of loss of neurons and dendrites caused by inflammatory and oxidative stress processes, the attenuation of Aβ-induced oxidative stress and neurotoxicity and the reduction of amyloid plaque burden (20, 34). Most, if not all, of these activities are related to the oxidative and nitrosative stress that is thought to play an essential role in the pathology of AD. The findings of this study can therefore also help us gain insight on a pathological level on the role of oxidative stress in early AD dementia patients.
Edaravone has been investigated in several other indications. An intravenous formulation was approved in Japan (Radicut®) as a therapeutic agent to reduce neuronal damage following acute ischemic stroke. Where it ameliorates ischemic neuronal damage and thereby preventing oxidative damage to brain cells. In addition, edaravone has been approved by the FDA under the trade name Radicava® for treatment of amyotrophic lateral sclerosis (ALS), a rare neurodegenerative disease. It was originally approved in 2017 with an intravenous administration, but in 2022 an oral formulation was also approved, Radicava ORS®. In ALS, it has been shown that edaravone reduces oxidative stress biomarker 3-NT in the CSF and that treatment with edaravone can significantly delay the progression as measured per the ALS functional rating scale (35). Treeway B.V. developed a new, patient-friendly oral formulation of edaravone, TW001. This formulation is currently in a Pivotal Phase III Clinical Trial in Europe for the treatment of ALS (ADORE; NCT05178810). In AD, it is anticipated that oral TW001 could provide a convenient daily at-home dosing for AD patients and caregivers with the goal to delay disease progression. Because it has been shown that edaravone can penetrate the blood-brain-barrier to exert its function and it can reduce oxidative stress in other neurodegenerative diseases, it is believed that it can also have a beneficial effect in AD patients.
Implementation of sensitive biomarkers to investigate innovative DMTs should allow for exploratory trials to be of limited duration (3 to 6 months) while still being informative and indicative of disease modification. A composite biomarker could even be more beneficial in this case as it takes into consideration the complexity of the disease and might result in a more sensitive biomarker able to detect changes in the context of this trial. Considering the complexity of AD, a broad range of biomarkers will be evaluated in this trial and multivariate modelling will determine the ultimate selection of biomarkers to be used in the future. This ultimate selection of biomarkers will thus only take place after completion of the clinical validation of these biomarkers in AD patients in the ASURE trial.
Exploratory efficacy endpoints focusing on cognition have also been included in this trial, these tests include the Clinical Dementia Rating scale - sum of boxes (CDR-SB) and the Cognitive Functional Composite (CFC). The sensitivity of the CFC to detect changes over a relatively short time period in early AD stages and its monitoring ability, make it highly fit for this trial. The use of the CFC also shows the collaborative nature of this trial between academia and biotech. The trail design is a combination expertise on AD from the Alzheimer Center Amsterdam as well expertise on edaravone and oxidative stress from Treeway. Furthermore, Treeway has discussed this full trial design during an Innovation Task Force meeting with the EMA, in particular the innovative biomarkers and how they can be further developed and contribute to improved future clinical trial designs.
In conclusion, the ASURE trial is combining well-known and well-established biomarkers with newer ones together with the goal to develop a composite biomarker and includes an innovative cognitive outcome within a relatively short trial.
References
Organization, W. h. Global action plan on the public health response to dementia, 2017.
Scheltens, P. et al. Alzheimer’s disease. Lancet 2021;397, 1577–1590, doi:https://doi.org/10.1016/S0140-6736(20)32205-4.
Jack, C. R. et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia 2018;14, 535–562, doi: https://doi.org/10.1016/j.jalz.2018.02.018
Parolo, S., Mariotti, F., Bora, P., Carboni, L. & Domenici, E. Single-cell-led drug repurposing for Alzheimer’s disease. Scientific Reports 2023;13, 222, doi:https://doi.org/10.1038/s41598-023-27420-x.
Tönnies, E. & Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. Journal of Alzheimer’s Disease 2017;57, 1105–1121, doi:https://doi.org/10.3233/JAD-161088.
Shelat, P. B. et al. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. Journal of Neurochemistry 2008;106, 45–55, doi:https://doi.org/10.1111/j.1471-4159.2008.05347.x.
Cheignon, C. et al. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biology 2018;14, 450–464, doi:https://doi.org/10.1016/j.redox.2017.10.014.
Carrillo-Mora, P., Luna, R. & Colín-Barenque, L. Amyloid beta: multiple mechanisms of toxicity and only some protective effects? Oxid Med Cell Longev 2014, 795375, doi:https://doi.org/10.1155/2014/795375.
Butterfield, D. A., Drake, J., Pocernich, C. & Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med 2001;7, 548–554, doi:https://doi.org/10.1016/s1471-4914(01)02173-6.
Sutherland, G. T., Chami, B., Youssef, P. & Witting, P. K. Oxidative stress in Alzheimer’s disease: Primary villain or physiological by-product? Redox Report 2013;18, 134–141, doi:https://doi.org/10.1179/1351000213Y.0000000052.
Su, B. et al. Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neuroscience Letters 2010;468, 267–271, doi:https://doi.org/10.1016/j.neulet.2009.11.010.
Bai, R., Guo, J., Ye, X.-Y., Xie, Y. & Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Research Reviews 2022;77, 101619, doi:https://doi.org/10.1016/j.arr.2022.101619(2022).
Doig, A. J. Positive Feedback Loops in Alzheimer’s Disease: The Alzheimer’s Feedback Hypothesis. J Alzheimers Dis 2018;66, 25–36, doi:https://doi.org/10.3233/jad-180583.
Yamamoto, Y., Kuwahara, T., Watanabe, K. & Watanabe, K. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one. Redox Rep 1996;2, 333–338, doi:https://doi.org/10.1080/13510002.1996.11747069.
Watanabe, K., Tanaka, M., Yuki, S., Hirai, M. & Yamamoto, Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr 2018;62, 20–38, doi:https://doi.org/10.3164/jcbn.17-62.
Fujisawa, A. & Yamamoto, Y. Edaravone, a potent free radical scavenger, reacts with peroxynitrite to produce predominantly 4-NO-edaravone. Redox Rep 2016;21, 98–103, doi:https://doi.org/10.1179/1351000215y.0000000025.
Pérez-González, A. & Galano, A. On the Outstanding Antioxidant Capacity of Edaravone Derivatives through Single Electron Transfer Reactions. The Journal of Physical Chemistry B 2012;116, 1180–1188, doi:https://doi.org/10.1021/jp209930y.
Pérez-González, A. & Galano, A. Ionization energies, proton affinities, and pKa values of a large series of edaravone derivatives: implication for their free radical scavenging activity. J Phys Chem B 2011;115, 10375–10384, doi:https://doi.org/10.1021/jp2047163.
Jiao, S. S. et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc Natl Acad Sci U S A 2015;112, 5225–5230, doi:https://doi.org/10.1073/pnas.1422998112.
Yan, Y. et al. Protective effect of edaravone against Alzheimer’s disease-relevant insults in neuroblastoma N2a cells. Neurosci Lett 2012;531, 160–165, doi:https://doi.org/10.1016/j.neulet.2012.10.043.
Zhang, L. et al. Edaravone reduces Aβ-induced oxidative damage in SH-SY5Y cells by activating the Nrf2/ARE signaling pathway. Life Sci 2019;221, 259–266, doi:https://doi.org/10.1016/j.lfs.2019.02.025.
Parikh, A. et al. Self-nanomicellizing solid dispersion of edaravone: part II: in vivo assessment of efficacy against behavior deficits and safety in Alzheimer’s disease model. Drug Des Devel Ther 2018;12, 2111–2128, doi:https://doi.org/10.2147/dddt.S161944.
Jutten, R. J. et al. The Cognitive-Functional Composite is sensitive to clinical progression in early dementia: Longitudinal findings from the Catch-Cog study cohort. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2020;6, e12020, doi:https://doi.org/10.1002/trc2.12020.
Wu, L. L., Chiou, C.-C., Chang, P.-Y. & Wu, J. T. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clinica Chimica Acta 2004;339, 1–9, doi:https://doi.org/10.1016/j.cccn.2003.09.010.
Mecocci, P. et al. Lymphocyte Oxidative DNA Damage and Plasma Antioxidants in Alzheimer Disease. Archives of Neurology 2002;59, 794–798, doi:https://doi.org/10.1001/archneur.59.5.794.
Abe, T., Tohgi, H., Isobe, C., Murata, T. & Sato, C. Remarkable increase in the concentration of 8-hydroxyguanosine in cerebrospinal fluid from patients with Alzheimer’s disease. Journal of Neuroscience Research 2002;70, 447–450, doi:https://doi.org/10.1002/jnr.10349.
Butterfield, D. A. et al. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: Implications for the role of nitration in the progression of Alzheimer’s disease. Brain Research 2007;1148, 243–248, doi:https://doi.org/10.1016/j.brainres.2007.02.084.
Jutten, R. J. et al. A composite measure of cognitive and functional progression in Alzheimer’s disease: Design of the Capturing Changes in Cognition study. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2017;3, 130–138, doi:https://doi.org/10.1016/j.trci.2017.01.004.
Jutten, R. J. et al. Detecting functional decline from normal aging to dementia: Development and validation of a short version of the Amsterdam IADL Questionnaire. Alzheimers Dement (Amst) 2017;8, 26–35, doi:https://doi.org/10.1016/j.dadm.2017.03.002.
Teng, E. et al. Cross-sectional and longitudinal assessments of function in prodromal-to-mild Alzheimer’s disease: A comparison of the ADCS-ADL and A-IADL-Q scales. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 2023;15, e12452, doi:https://doi.org/10.1002/dad2.12452.
Dubbelman, M. A. et al. The influence of diversity on the measurement of functional impairment: An international validation of the Amsterdam IADL Questionnaire in eight countries. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 2020;12, e12021, doi:https://doi.org/10.1002/dad2.12021.
Dubbelman, M. A. et al. Determining the Minimal Important Change of Everyday Functioning in Dementia: Pursuing Clinical Meaningfulness. Neurology 2022;99, e954–e964, doi:https://doi.org/10.1212/wnl.0000000000200781.
Jutten, R. J. et al. Assessing cognition and daily function in early dementia using the cognitive-functional composite: findings from the Catch-Cog study cohort. Alzheimer’s Research & Therapy 2019;11, 45, doi:https://doi.org/10.1186/s13195-019-0500-5.
Zhang, N. et al. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke 2005;36, 2220–2225, doi:https://doi.org/10.1161/01.Str.0000182241.07096.06.
Yoshino, H. & Kimura, A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study). Amyotrophic Lateral Sclerosis 2006;7, 247–251, doi:https://doi.org/10.1080/17482960600881870.
Funding
Funding: This trial is kindly sponsored by the Alzheimer’s Drug Discovery Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures: MO has received consultancy fees from New Amsterdam Pharma (paid to the institute). PvB declares that he has no competing interests. PvB is an advisory consultant for several pharmaceutical companies in the field of neurodegeneration. Research of CET is supported by the European Commission (Marie Curie International Training Network, grant agreement No 860197 (mIRIADE), Innovative Medicines Initiatives 3TR (Horizon 2020, grant no 831434) EPND (FMI 2 Joint Undertaking (JU), grant No. 101034344) and JPND (bPRIDE), National MS Society (Progressive MS alliance), Alzheimer Association, Health Holland, the Dutch Research Council (ZonMW), Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands. CT is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health-Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). CET has a collaboration contract with ADx Neurosciences, Quanterix and Eli Lilly, performed contract research or received grants from AC-Immune, Axon Neurosciences, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, PeopleBio, Roche, Siemens, Toyama, Vivoryon. CET serves on editorial boards of Medidact Neurologie/Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology & Neuroinflammation. EGBV has received consultancy fees (paid to institute) for New Amsterdam Pharma, Treeway, ReMynd, Vivoryon, Biogen, Vigil Neuroscience, ImmunoBrain Checkpoint en Roche. Within his university affiliation he is PI of studies of DIAN, AC immune, Alnylam, CogRX therapeutics, New Amsterdam Pharma, Janssen, UCB, Roche, Vivoryon, ImmunoBrain, GemVax and Alector. Sub-Investigator from Eli Lilly, Biogen en Fuij Film Toyama. Co-founder van het CANDIDATE Center Amsterdam UMC.
Ethical standards: The study will be performed in accordance with the Declaration of Helsinki adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and last amended by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013. The trial will meet the ethical requirements set in the Directive 2001/20/EC of the European Parliament and of the Council of 04-Apr-2001, which will be replaced by Regulation (EU) No 536/2014 on 31-Jan-2022.1.
Rights and permissions
Open Access : This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Oosthoek, M., Lili, A., Almeida, A. et al. ASURE Clinical Trial Protocol: A Randomized, Placebo-Controlled, Proof-of-Concept Study Aiming to Evaluate Safety and Target Engagement following Administration of TW001 in Early Alzheimer’s Disease Patients. J Prev Alzheimers Dis 10, 669–674 (2023). https://doi.org/10.14283/jpad.2023.107
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2023.107