Abstract
As research evolves in prodromal AD, the need to validate sufficiently sensitive outcome measures, e.g. the Alzheimer’s Disease Composite Score (ADCOMS) is clear. In the LipiDiDiet randomized trial in prodromal AD, cognitive decline in the study population was much less than expected in the timeframe studied. While the primary composite endpoint was insufficiently sensitive to detect a difference in the modified intention to treat population, the per-protocol population showed less decline in the active than the control group, indicating better treatment effects with regular product intake. These results were further strengthened by significant benefits on secondary endpoints of cognition and function, and brain atrophy. The present post-hoc analysis investigated whether ADCOMS could detect a difference between groups in the LipiDiDiet population (138 active, 140 control). The estimated mean change in ADCOMS from baseline (standard error) was 0.085 (0.018) in the active and 0.133 (0.018) in the control group; estimated mean treatment difference −0.048 (95% confidence intervals −0.090, −0.007; p=0.023), or 36% less decline in the active group. This suggests ADCOMS identified the cognitive and functional benefits observed previously, confirming the sensitivity of this composite measure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prodromal Alzheimer’s disease (AD) is characterized by mild cognitive and functional impairment with defined changes in specific biomarkers (1–3). The LipiDiDiet trial was one of the first randomized clinical trials conducted in subjects with prodromal AD who were selected using the clinical and biomarker-based criteria originally described by Dubois et al. (1). The trial investigated the effects of the specific nutrient combination Fortasyn Connect (Souvenaid) on cognitive, functional, and other disease related parameters in this population (4). We previously reported that the intervention had no significant effect in the primary analysis on the 2-year primary endpoint, a 5-item neuropsychological test battery (NTB), yet significant differences for this endpoint were found in the pre-defined secondary analysis of the per-protocol population and the pre-defined subgroup analysis (4). Of note, in this trial population, the rate of cognitive decline as measured by the NTB score was several times less than expected, which means that the primary endpoint was insufficiently sensitive to detect a difference between the interventional and control groups (4). While such an observation adds important information about the early clinical course of prodromal AD (5, 6), it clearly highlights the ongoing need for more sensitive tools to detect changes in cognitive performance in this population.
Evaluating the effects of interventions for mildly affected populations with only limited cognitive and functional decline and subtle impairment such as subjects with prodromal AD, requires the use of sufficiently sensitive and informative composite outcome measures. The Clinical Dementia Rating — Sum of Boxes (CDR-SB) has been proposed as such a measure (7). More recently, the Alzheimer’s Disease Composite Score (ADCOMS) was developed as a broader composite clinical outcome measure for trials in prodromal and mild AD dementia (8). ADCOMS consists of cognitive and functional items from three commonly used scales in AD dementia trials: the Alzheimer’s Disease Assessment Scale — cognitive subscale (ADAS-cog), Mini-Mental State Examination (MMSE), and CDR-SB. In subjects with early AD, the combination of selected items from these scales was shown to have the highest sensitivity for measuring changes and intervention effects over time compared with the individual scales (8). Preliminary results from the first randomized controlled trial using ADCOMS as the primary outcome measure were interpreted as supporting the applicability of this composite score in subjects with early AD (9). However, more studies are needed to establish general applicability across different trial settings and the contribution of the different subdomains to the composite.
ADCOMS has been proposed as a new standard outcome measure for trials in prodromal AD; therefore, we did a post-hoc analysis of data from the LipiDiDiet trial primarily to compare Fortasyn Connect and control groups using ADCOMS and its subdomains as a potentially more sensitive measure of intervention effects than the NTB used in the primary analysis. An additional aim of the analysis was to use data from subjects with prodromal AD to provide broader knowledge of ADCOMS as a single clinical outcome measure in early AD trials.
Subjects and methods
Detailed methods for the LipiDiDiet trial (Netherlands Trial Registry NTR1705) were published previously (4). In summary, LipiDiDiet was a 24-month, double-blind, parallel-group, multi-center randomized controlled trial (11 sites in Finland, Germany, the Netherlands, and Sweden), with optional 12-month double-blind extensions. Eligible participants with prodromal AD, defined according to the International Working Group (IWG)-1 criteria (1), were randomly assigned (1:1) to active intervention (once-daily 125 mL drink containing the multinutrient combination Fortasyn Connect provided by Nutricia [Zoetermeer, the Netherlands]) or a same-taste iso-caloric control product. The primary outcome was the change in a cognitive function composite z-score based on five items of an NTB. CDR-SB was a secondary outcome while ADAS-cog-13 and MMSE were exploratory parameters. Participants provided written consent and the trial was approved by ethics committees of all sites and done in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines.
We used the LipiDiDiet trial data to do a post-hoc analysis of outcomes included in the ADCOMS tool, which consists of four ADAS-Cog subscale items (delayed word recall, orientation, word recognition, and word finding difficulty), two MMSE items (orientation time and drawing), and all six CDR-SB items (personal care, community affairs, home and hobbies, judgement and problem solving, memory, and orientation), as described previously by Wang and colleagues (8).
In this analysis, ADCOMS scores were calculated using the selected 12 items and corresponding partial least squares coefficients. Composite scores range from 0.0 to a maximum of 1.97, where higher values indicate worse performance. The contribution of the separate subdomains (ADAS-cog, MMSE, and CDR-SB) to the total score was explored by calculating the separate domains based on the same items and coefficients. Total ADCOMS scores and subdomain scores were calculated only if subject data were available for all 12 items. Statistical analyses were performed as planned using linear mixed models for repeated measures with real measurement time as continuous variable (primary model) or planned visit time as categorical variable (planned sensitivity model) in a modified intention-to-treat (mITT) population of all participants randomly assigned, excluding data after the start of rescue medication (defined as use of active product or Alzheimer’s disease medication after dementia diagnosis). Further details about these statistical models were described previously (4). Additional sensitivity analyses using the primary and sensitivity models with baseline in the outcome vector, a 2-sided, independent t-test, and a non-parametric Mann-Whitney U test were performed to test the robustness of results. Effect sizes were reported using Cohen’s d standardized effect size calculated based on the mean treatment difference over 24 months, estimated in the mixed model and pooled SD based on the sample size at the 24-month visit. Similar analyses were also done on a per-protocol dataset excluding participants with major protocol deviations.
Results
This analysis includes data obtained from 311 participants with prodromal AD (153 active group and 158 control group) enrolled between April 20, 2009, and July 3, 2013. In the mITT population, data were available for the post-hoc ADCOMS analysis from 278 participants (138 active and 140 control) at baseline, 225 (109 active and 116 control) at month 12, and 164 (73 active and 91 control) at month 24, which is comparable to the data available for the mITT analysis of the NTB primary outcome in the original paper (4).
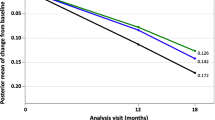
ADCOMS scores at baseline were 0.258 (standard deviation [SD] 0.143, n=138) in the active group and 0.247 (SD 0.140, n=140) in the control group (Table 1a). Figure 1 shows changes in ADCOMS scores and subdomain scores during the 24-month intervention period. While both groups showed higher ADCOMS scores over time, worsening was 36% less in the active group than in the control group (Figure 1A). The estimated mean change from baseline (standard error) was 0.085 (0.018) in the active group and 0.133 (0.018) in the control group; the corresponding estimated mean treatment difference was −0.048 (95% confidence intervals −0.090 to −0.007; p=0.023). Analysis of the ADCOMS subdomains (Figures 1B-D) showed that the difference between active and control groups was greatest for the six-item CDR-SB subdomain (34% less worsening) and the 2-item MMSE subdomain (63% less worsening). The estimated mean change from baseline (standard error) was 0.065 (0.016) in the active group and 0.099 (0.016) in the control group for the sixitem CDR-SB subdomain (p=0.033), and 0.007 (0.005) in the active group and 0.019 (0.005) in the control group for the 2-item MMSE subdomain (p=0.065). No differences between groups were observed for the 4-item ADAS-cog subdomain. The planned sensitivity analysis showed significant differences between groups over 24 months in worsening of ADCOMS scores (p=0.023) and worsening of six-item CDR-SB (p=0.032), while there was a trend on the 2-item MMSE (p=0.068) and no difference on the 4-item ADAS-cog (p=0.499). The additional sensitivity analyses on ADCOMS and subdomains confirmed the results (ADCOMS: primary model with baseline in the outcome vector, p=0.038; t-test, p=0.059; Mann-Whithney U test, p=0.036).
Per-protocol analysis including baseline data from 257 participants (129 active and 128 control) confirmed the findings in the mITT analysis (Table 1b).
Effect size analyses of changes from baseline over 24 months on ADCOMS score showed Cohen’s d values of 0.31 in the mITT population and 0.39 in the per-protocol population, indicating a small to medium effect in the active group (10). Effect sizes >0.2 were also observed for the MMSE and CDR-SB subdomains in the mITT (0.27 and 0.25, respectively) and per-protocol (0.25 and 0.33, respectively) analyses.
Discussion
Research practice in subjects with prodromal AD is still evolving, and since the 24-month LipiDiDiet trial database was locked, there has been a growing recognition that combined cognitive-functional measurement tools may provide a more sensitive way to assess the efficacy of novel interventions than those currently available (7, 11). To reflect contemporary research practice, we used ADCOMS in a post-hoc analysis of the LipiDiDiet trial data and found a significant intervention effect for Fortasyn Connect over 24 months in subjects with prodromal AD. The active group showed significantly less clinical decline over 24 months as measured by ADCOMS, and this effect was driven largely by differences in the CDR-SB and MMSE subdomains. We previously reported a significant benefit for Fortasyn Connect using CDR-SB and showed that stabilization of CDR-SB scores was more pronounced with increasing baseline MMSE (4), which supports the notion that early rather than late treatment within the prodromal phase of dementia may lead to better outcomes when using CDR-SB as a cognitive-functional measure. ADCOMS data in this post-hoc analysis (data not shown) also suggest that earlier intervention is associated with better outcomes for Fortasyn Connect.
The ADCOMS score is weighted toward the CDR-SB which functions as the framework of the score, but only takes on values from 0.5 to 7 (in increments of 0.5) for the majority of participants. The MMSE and ADAS-cog items provide further discriminatory ability between these seven points, enhancing the performance of the scale, but not performing as reliably when isolated. The inclusion of multiple measures of important cognitive domains stabilizes estimates and protects against spurious results. The CDR-SB has historically been more sensitive to progression, but less sensitive to treatment effects due to low variability, contrasted with cognitive scales which have been more sensitive to treatment effects but also highly variable. The weighted combination was designed to combine changes between points on the CDR-SB with detailed changes in cognitive items, with the sensitive items potentially differing from one study to another. In this case, the CDR-SB items and the MMSE items were sensitive to changes, and the ADAS-cog items were less sensitive, allowing the ADCOMS scale to detect treatment related changes due to both functional and cognitive contributions.
The effect size analysis reported here indicates that the magnitude of the intervention effect measured using ADCOMS was large enough to be clinically detectable. The effect size for ADCOMS (Cohen’s d 0.31) was similar to the value previously reported for CDR-SB (0.33) (4). The magnitude of the intervention effects seen with ADCOMS and CDR-SB, both in this analysis and the original trial report (4), were more pronounced in the per-protocol analysis, possibly reflecting the importance of long-term protocol adherence.
These results should be interpreted with caution because of the post-hoc nature of the analysis with a relatively new cognitive-functional measurement tool. Nevertheless, ADCOMS was developed using robust methodology (8), and these analyses further contribute to the validation of ADCOMS in clinical trials in subjects with early AD and suggest applicability and sensitivity across different intervention strategies in the earliest stages of dementia. Our post-hoc ADCOMS analyses are consistent with the overall findings from the LipiDiDiet trial (4) and in combination with data from other authors (8), provide further evidence that ADCOMS, a broad measure of cognitive function, may be useful over a range of interventions and trial designs in early AD.
In conclusion, this analysis suggests that the cognitive and functional benefits observed in the LipiDiDiet trial were also identified using ADCOMS, adding to the accumulating evidence validating this sensitive and broad composite outcome measure in prodromal AD trials.
Change history
23 September 2019
The article «Alzheimer’s Disease Composite Score: a Post-Hoc Analysis Using Data from the LipiDiDiet Trial in Prodromal Alzheimer’s Disease», written by S.B. Hendrix, H. Soininen, A.M.J. van Hees, N. Ellison, P.J. Visser, A. Solomon, A. Attali, K. Blennow, M. Kivipelto, T. Hartmann, was originally published electronically on the publisher’s internet portal (currently SpringerLink) on 10 September 2019 without open access. With the author(s)’ decision to opt for Open Choice the copyright of the article changed to © The Author(s) 2019 and the article is forthwith distributed under the terms of the Creative Commons Attribution 4.0 International License (<ExternalRef><RefSource>http://creativecommons.org/licenses/by/4.0/</RefSource><RefTarget Address="http://creativecommons.org/licenses/by/4.0/" TargetType="URL"/></ExternalRef>), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The original article was corrected.
References
Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746.
Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629.
Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323.
Soininen H, Solomon A, Visser PJ, et al. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16:965–975.
Hamel R, Kohler S, Sistermans N, et al. The trajectory of cognitive decline in the pre-dementia phase in memory clinic visitors: findings from the 4C-MCI study. Psychol Med. 2015;45:1509–1519.
Ellis KA, Szoeke C, Bush AI, et al. Rates of diagnostic transition and cognitive change at 18-month follow-up among 1,112 participants in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL). Int Psychogeriatr. 2014;26:543–554.
U.S. Department of Health and Human Services Food and Drug Administration. Early Alzheimer’s Disease: Developing Drugs for Treatment Guidance for Industry (Draft Guidance) 2018. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf.
Wang J, Logovinsky V, Hendrix SB, et al. ADCOMS: a composite clinical outcome for prodromal Alzheimer’s disease trials. J Neurol Neurosurg Psychiatry. 2016;87:993–999.
Swanson CJ, Zhang Y, Dhadda S, et al. Treatment of early AD subjects with BAN2401, an anti-Aβ protofibril monoclonal antibody, significantly clears amyloid plaque and reduces clinical decline. Alzheimer’s Association International Conference; 20–26 July 2018; Chicago, USA2018. p. Abstract ID: 27531.
Cohen J. Statistical power analysis for the behavioral sciences (Second edition). 2 ed: Lawrence Erlbaum Associates; 1988.
Vellas B, Bateman R, Blennow K, et al. Endpoints for pre-dementia AD trials: A report from the EU/US/CTAD Task Force. J Prev Alzheimers Dis. 2015;2:128–135.
Acknowledgments
We thank all participants enrolled in the study and their families; all members of the LipiDiDiet clinical study group; all investigators and on-site study staff for their efforts in the conduct of the field work.
Funding
Funding: The research leading to these results was mainly funded by the European Commission under the 7th framework program of the European Union (grant agreement number 211696). Additional funding was provided by the EU Joint Program — Neurodegenerative Disease Research (MIND-AD grant); Kuopio University Hospital, Finland (EVO/VTR grant); and Academy of Finland (grant 287490). These funders had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript. This post-hoc analysis was funded by Danone Nutricia Research and performed by Pentara Corporation. The corresponding author had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: SBH and NNE report financial compensation for statistical analysis from Danone Nutricia Research. HS reports personal fees from ACImmune and MERCK, outside the submitted work. AMJH and AA are employees of Danone Nutricia Research. PJV reports grants from Inn ovative Medicine Initiative and ZonMw, during the conduct of the study, and non-financial support from GE Healthcare and grants from Biogen, outside the submitted work. AS reports grants from Academy of Finland, during the conduct of the study, and grants from Alzheimerfonden Sweden and Stockholm County Council (ALF), outside the submitted work. MK reports grants from EU Joint Program - Neurodegenerative Disease Research (MIND-AD), during the conduct of the study, and grants from Alzheimerfonden Sweden, Stockholm County Council (ALF), Academy of Finland, Swedish Research Council, Knut and Alice Wallenberg Foundation, Center for Innovative Medicine at Karolinska Institutet, Sweden, and Stiftelsen Stockholms Sjukhem, Sweden, outside the submitted work. TH reports grants from EU FP7 (LipiDiDiet), EU Joint Program — Neurodegenerative Disease Research (MIND-AD), and Danone Nutricia Research (LipiDiDiet Extension), during the conduct of the study. KB has nothing to disclose.
Ethical standards: The study was approved by ethics committees of all sites and done in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines.
Additional information
A correction to this article is available at https://doi.org/10.1007/s42414-019-0001-5
Rights and permissions
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Hendrix, S.B., Soininen, H., van Hees, A.M.J. et al. Alzheimer’s Disease Composite Score: a Post-Hoc Analysis Using Data from the LipiDiDiet Trial in Prodromal Alzheimer’s Disease. J Prev Alzheimers Dis 6, 232–236 (2019). https://doi.org/10.14283/jpad.2019.33
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2019.33