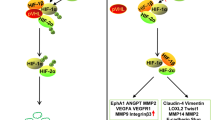
Abstract
In this article we consider the factors responsible for the unique nature of the pericellular matrix of solid tumors and we discuss the role of alterations of tumor blood vessel structure. We examine the role of VEGF (vascular endothelial growth factor), a factor controlling permeability of capillaries, plasma protein extravasation, and the formation of a fibrin barrier. We discuss how this barrier could be destroyed by metalloproteinases bound on the surface of endothelial cells migrating through the matrix and how these enzymes are responsible for the activation of gelatinases that destroy basement membranes. The process called tubulogenesis, which gives rise to hyperpermeable tumor capillaries, will also be described. Alterations of the blood vessel structure leading to hypoxia of the matrix, and accumulation of plasma proteins and of blood cells will be treated. Finally, we review some of the strategies that might exploit this knowledge about the nature of the tumoral matrix for designing novel anticancer treatments.
Similar content being viewed by others
References
Brown, J.M. and Giaccia, A.J. (1998) The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 58, 1408–1416.
Grunt, T.W., Lametschwandtner, A., and Staindl, O. (1995) The vascular pattern of basal cell tumors: light microscopy and scanning electron microscopy study on vascular corrosion casts. Microvasc. Res. 29, 371–386.
Dewhirst, M.W., Tso, C.Y., Oliver, R., Gustafson, C.S., Socomb, T.W., and Gross, J.F. (1989) Morphologic and hemodynamic comparison of tumor and healing normal tissue microvasculature. Int.J.Radiat.Oncol.Biol.Phys. 17, 91–99.
Gullino, P.M. (1996) The internal milieu of tumors. Prog. Exp. Tumor Res. 8, 1–25.
Gullino, P.M., Ziche, M., and Alessandri, G. (1990) Gangliosides, copper ions and angiogenic capacity of adult tissues. Cancer Metastasis Rev. 9, 239–251.
Alessandri, G., Cornaglia-Ferraris, P., and Gullino, P.M. (1997) Angiogenic and angiostatic microenvironment in tumors. Role of gangliosides. Acta Oncol. 36, 383–387.
Srinivas, V., Zhu, X., Salceda, S., Nakamura, R., and Caro, J. (1998) Hypoxia -inducible factor (HIF-1alpha) is a non-heme iron protein. Implications for oxigen sensing. J. Biol. Chem. 273, 18019–18022.
Chiarugi, V., Magnelli, L., Chiarugi, A., and Gallo, O. (1999) Hypoxia induces pivotal tumor angiogenesis control factors including p53, vascular endothelial growth factor, and the NFkappaB-dependent inducible nitric oxide synthase and cyclooxygenase-2. J. Cancer Res. Clin. Oncol. 125, 525–528.
Blancher, C. and Harris A.L. (1998) The molecular basis of the hypoxia response patway: tumor hypoxia as a therapy target. Cancer Metastasis Rev. 17, 187–194.
Guillemin, K. and Krasnow, M.A. (1997) The hypoxic response: huffing and HIFing. Cell 89, 9–12.
Tsuji, M., Kawano, S., Tsuji, S., Sawaoka, H., Hori, M. and Dubois, R.N. (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93, 705–716.
Korpelainen, E.I., and Alitalo, K. (1998) Signaling angiogenesis and lymphangiogenesis. Curr. Opin. Cell.Biol. 10, 159–164.
Hiraoka, N., Allen, E., Apel, I.J., Gyetko, M.R., and Weiss, S.J. (1998) Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 95, 365–377.
Zucker, S., Mirza, H., Conner, C., Lorenz, A.F., Drews, M.H., Bahou, W.F. and Jesty, J. (1998) Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int. J. Cancer 75, 780–786.
Bellamy, W.T., Richter, L., Frutiger, Y., and Gronan, T.M. (1999) Expression of vascular endothelial growth factor and its receptors in hematopoietic malignacies. Cancer Res. 59, 728–733.
Forsyth, P.A., Wong, H., Laing, T.D., et al. (1999) Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of pathophysiology of malignant gliomas. Br. J. Cancer 79, 1828–1835.
Yabkowitz, R., Meyer, S., Black, T., Elliot, G., Merewhether, L.A. and Yamane, H.K. (1999) Inflammatory cytokines and vascular endothelial growth factor stimulate the release of soluble tie receptor from human endothelial cells via metalloproteinase activation. Blood 15, 1969–1979.
Forsyth, P.A., Laing, T.D., Gibson, A.W., Rewcastle, N.B., Brasher, P., Sutherland, G., Johnston, R.N. and Edwards, D.R. (1998) High level of gelatinase-B and active gelatinase-A in metastatic glioblastoma. J. Neurooncol. 36, 21–29.
Ueno, H., Nakamura, H., Inoue, M., et al. (1998) Expression and tissue localization of membrane types 1,2 and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 57, 2055–2060.
Basbaum, C.B., and Werb, Z. (1996) Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr. Opin. Cell. Biol. 8, 731–738.
Werb, Z. (1997) ECM and cell surface proteolysis: regulating cellular ecology. Cell 91, 439–442.
Brown, L.F., Berse, B., Tognazzi, K., et al. (1992) Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int. 42, 1457–1461.
Brown, L.F., Detmar, M., Tognazzi, K., Abu-Jawdeh, G., and Iruela-Arispe, M.L. (1997) Uterine smooth muscle cells express functional receptors (flt-1 and KDR) for vascular permeability factor/vascular endothelial growth factor. Lab. Invest. 76, 245–255.
Dvorak, H.F., Brown, L.F., Detmar, M., and Dvorak, A.M. (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146, 1029–1039.
Brown, L.F., Van de Water, L., Harvey, V.S., and Dvorak, H.F. (1998) Fibrinogen influx and accumulation of cross-linked fibrin in healing wounds and tumor stroma. Am.J.Pathol. 130, 455–465.
Murphy, G., and Knauper, V. (1997) Relating matrix metalloproteinase structure to function. Why the “hemopexin” domain? Matrix Biol. 15, 511–518.
Pendas, A.M., Knauper, V., Puente, X.S., Llano, E., Mattei, M.G., Apte, S., Murphy, G., and Lopez-Otin, C. (1997) Identification and characterization of a novel human matrix metalloproteinase witj unique structural characteristic, chromosomal location and tissue distribution. J. Biol. Chem. 272, 4281–4286.
Puente, X.S., Pendas, A.M., Llano, E., Velasco, G., and Lopez-Otin, C. Molecular cloning of a novel membrane-type matrix metalloproteinase from a human breast carcinoma. Cancer Res. 56, 944–949.
Atkinson, S.J., Crabbe, T., Cowell, S., et al. (1995) Intermolecular autolytic cleavage can contribute to the activation of progelatinase A by cell membranes. J. Biol. Chem. 270, 30679–30685.
Nguyen, M., Arkell, J, and Jackson, C.J. (1999) Thrombin rapidly and efficiently activates gelatinase A in human microvascular endothelial cells via a mechanism indipendent of active MT1 matrix metalloproteinase. Lab.Invest. 79, 467–475.
Herbert, J.M., Lamarche, I., and Carmeliet, P. (1997) Urokinase and tissue-type plasminogen activator are required for the mitogenic and chemotactic effects of bovine fibroblast growth factor and platelet-derived growth factor-BB for vascular smooth muscle cells. J.Biol. Chem. 272, 23585–23591.
Risau, W. (1997) Mechanisms of angiogenesis. Nature 386, 671–674.
Sato, A., Yamamoto, E., and Seiki, M. A. (1994) A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature 370, 61–65.
Maniotis, A.J., Folberg, R., Hess, A., et al. (1999) Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J.Pathol. 155, 739–752.
Foldberg, R., Hendrix, M.J., and Maniotis, A.J. (2000) Vasculogenic mimicry and tumor angiogenesis. Am.J. Pathol. 156, 361–381.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chiarugi, V., Ruggiero, M. & Magnelli, L. Angiogenesis and the unique nature of tumor matrix. Mol Biotechnol 21, 85–90 (2002). https://doi.org/10.1385/MB:21:1:085
Issue Date:
DOI: https://doi.org/10.1385/MB:21:1:085