Abstract
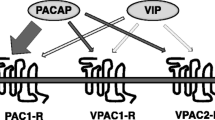
Receptors for vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) in guinea pig cerebral cortex were characterized by (1) radioreceptor binding of 125I-labeled VIP (human/rat/porcine), and (2) cyclic AMP (cAMP) formation. Saturation analysis of 125I-VIP binding to membranes of guinea pig cerebral cortex resulted in a linear Scatchard plot, suggesting the presence of a single class of high-affinity receptor-binding sites, with a Kd of 0.63 nM and a Bmax of 77 fmol/mg protein. Various peptides from the PACAP/VIP/secretin family displaced the specific binding of 125I-VIP to guinea pig cerebrum with the relative rank order of potency: chicken VIP (cVIP) ≥ PACP38 ∼ PACAP27 ∼ guinea pig VIP (gpVIP) ≥ mammalian (human/rat/porcine) VIP (mVIP) > peptide histidine-methionine (PHM) > peptide histidine-isoleucine (PHI) > secretin. Analysis of the competition curves revealed displacement of 125I-VIP from high- and lower-affinity binding sites, with IC50 values in the picomolar and the nanomolar range, respectively. About 70% of the specific 125I-VIP-binding sites in guinea pig cerebral cortex were sensitive to Gpp(NH)p, a nonhydrolyzable analog of GTP. Pituitary adenylate cyclase-activating polypeptide 38 (PACAP38), PACAP27, cVIP, gpVIP, mVIP, PHM, and PHI stimulated cAMP production in [3H]adenine-prelabeled slices of guinea pig cerebral cortex in a concentration-dependent manner. Of the tested peptides, the most effective were PACAP38 and PACAP27, which at a 1 µM concentration produced a 17- to 19-fold rise in cAMP synthesis, increasing the nucleotide production to approx 11% conversion above the control value. The three forms of VIP (cVIP, mVIP, and gpVIP) at the highest concentration used, i.e., 3 µM, produced net increases in cAMP production in the range of 8–9% conversion, whereas 5 µM PHM and PHI, by, respectively, 6.7% and 4.9% conversion. It is concluded that cerebral cortex of guinea pig contains VPAC- type receptors positively linked to cAMP formation. In addition, the observed stronger action of PACAP (both PACAP38 and PACAP27), when compared to any form of VIP, on cAMP production in this tissue, suggests its interaction with both PAC1 and VPAC receptors.
Similar content being viewed by others
References
Berisha H. I., Bratut M., Bangale Y., Colasurdo G., Paul S., and Said S. I. (2002) New evidence for transmitter role of VIP in the airways: impaired relaxation by a catalytic antibody. Pulm. Pharmacol. Ther. 15, 121–127.
Braas K. M., May V., Harakall S. A., Hardwick J. C., and Parsons R. L. (1998) Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J. Neurosci. 18, 9766–9779.
Christopoulos A. and Kenakin T. (2002) G protein-coupled receptor allosterism and complexing. Pharmacol. Rev., 54, 323–374.
Delgado M., Abad C., Martinez C., Juarranz M. G., Arranz A., Gomariz R. P., and Leceta J. (2002) Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J. Mol. Med. 80, 16–24.
Dietl M. M., Hof P. R., Martin J. L., Magistretti P. J., and Palacios J. M. (1990) Autoradiographic analysis of the distribution of vasoactive intestinal peptide binding sites in the vertebrate central nervous system: a phylogenetic study. Brain Res. 520, 14–26.
Du B. H., Eng J., Hulmes J. D., Chang M., Pan Y. C. E., and Yalow R. S. (1985) Guinea pig has a unique mammalian VIP. Biochem. Biophys. Res. Commun. 128, 1093–1098.
Eng J., Yu J., Rattan S., and Yalow R. S. (1992) Isolation and amino acid sequences of opossum vasoactive intestinal polypeptide and cholecystokinin octapeptide. Proc. Natl. Acad. Sci. U. S. A. 89, 1809–1811.
Gourlet P., Vandermeers-Piret M. C., and Waelbroeck M. (1998) Vasoactive intestinal peptide modification at position 22 allows discrimination between receptor subtypes. Eur. J. Pharmacol. 348, 95–99.
Gozes I. and Brenneman D. E. (2000) A new concept in the pharmacology of neuroprotection. J. Mol. Neurosci. 14, 61–68.
Gozes I. and Brenneman D.E. (1989) VIP: molecular biology and neurobiological function. Mol. Neurobiol. 3, 201–236.
Gozes I., Fridkin M., Hill J. M., and Brenneman D. E. (1999) Pharmaceutical VIP: prospects and problems. Curr. Med. Chem. 6, 1019–1034.
Harmar A.J., Arimura A., Gozes I., Journot L., Laburthe M., Pisegna J. R., et al. (1998) International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 50, 265–270.
Heinemann A. and Holzer P. (1999) Stimulant action of pituitary adenylate cyclase-activating peptide on normal and drug-compromised peristalsis in the guinea-pig intestine. Br. J. Pharmacol. 127, 763–771.
Hill J. M., Harris A., and Hilton-Clarke D.I. (1992) Regional distribution of guanine nucleotide-sensitive and guanine-insensitive vasoactive intestinal peptide receptors in rat brain. Neuroscience 48, 925–932.
Hill J. M., Lee S. J., Dibbern D. A. Jr., Fridkin M., Gozes I., and Brenneman D. E. (1999) Pharmacologically distinct vasoactive intestinal peptide binding sites: CNS localization and the role in embryonic growth. Neuroscience 93, 783–791.
Ito T., Hou W., Katsuno T., Igarashi H., Pradhan T. K., Mantey S. A., et al. (2000) Rat and guinea pig pancreatic acini possess both VIP1 and VIP2 receptors, which mediate enzyme secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G64-G74.
Kieffer T. J. and Habener J. F. (1999) The glucagon-like peptides. Endocr. Rev. 20, 876–913.
Klimaschewski L. (1997) VIP—a “very important peptide” in the sympathetic nervous system? Anat. Embryol. 196, 269–277.
Laburthe M., Convineau A., and Marie M.-C. (2002) VPAC receptors for VIP and PACAP. Recept. Channels 8, 137–153.
Linden A., Yoshihara S., Chan B., and Nadel J. A. (1995) Inhibition of bronchoconstriction by pituitary adenylate cyclase activating polypeptide (PACAP 1-27) in guinea-pigs in vivo. Br. J. Pharmacol. 115, 913–916.
Lowry O. H., Rosenbrough N. J., Farr A. L., and Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275.
Magistretti P. J., Cardinaux J. R., and Martin J. L. (1998) VIP and PACAP in the CNS: regulators of glial energy metabolism and modulators of glutamatergic signaling. Ann. N.Y. Acad. Sci. 865, 213–225.
Martin J. L., Feinstein D. L., Yu N., Sorg O., Rossier C., and Magistretti P. J. (1992) VIP receptor subtypes in mouse cerebral cortex: evidence for a differential localization in astrocytes, microvessels, and synaptosomal membranes. Brain Res. 587, 1–12.
Meunier A. C., Voisin P., Van Camp G., Cenatiempo Y., and Muller J. M. (1991) Molecular characterization and peptide specificity of two vasoactive intestinal peptide (VIP) binding sites in the chicken pineal. Neuropeptides 19, 1–8.
Miyata A., Arimura A., Dahl R. R., Minamino M., Uehara A., Jiang L., et al. (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 164, 567–574.
Moody T. W., Cha D., Fahrenkrug J., and Jensen R. T. (2003) Neuropeptides as autocrine growth factors in cancer cells. Curr. Pharm. Des. 9, 495–509.
Nicole P., Lins L., Rouyer-Fessard C., Drouot C., Fulcrand P., Thomas A., et al. (2000) Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a highly selective VPAC1 receptor agonist. J. Biol. Chem. 275, 24,003–24,012.
Nilsson A. (1974) Isolation, amino acid composition and terminal amino acid residues of the vasoactive octacosapeptide from chicken intestine. Partial purification of chicken secretin. FEBS Lett. 47, 284–289.
Nowak J. Z. and Kuba K. (2001) Vasoactive intestinal peptide-stimulated adenosine 3′,5′-cyclic monophosphate formation in cerebral cortex and hypothalamus of chick and rat: comparison of the chicken and mammalian peptide. Neurosci. Lett. 297, 93–96.
Nowak J. Z. and Sek B. (1994) Stimulatory effect of histamine on cyclic AMP formation in chick pineal gland. J. Neurochem. 63, 1338–1345.
Nowak J. Z. and Zawilska J. B. (2003) PACAP in avians: origin, occurrence and receptors—pharmacological and functional considerations. Curr. Pharm. Des. 9, 465–481.
Nowak J. Z., Kuba K., and Zawilska J. B. (1999) Stimulatory effect of pituitary adenylate cyclase-activating polypeptide (PACAP) on cyclic AMP formation in the hypothalamus and cerebral cortex of four avians and rat. Pol. J. Pharmacol. 51, 87–91.
Parsons R. L., Rossignol T. M., Calupca M. A., Hardwick J. C., and Braas K. M. (2000) PACAP peptides modulate guinea pig cardiac neuron membrane excitability and neuropeptide expression. Ann. NY Acad. Sci. 921, 202–210.
Pineau N., Lelievre V., Goursaud S., Hilairet S., Waschek J. A., Janet T., and Muller J.- M. (2001) The polypeptide PHI discriminates a GTP-insensitive form of vip receptor in liver membranes. Neuropeptides 35, 117–125.
Robberecht P., De Neef P., Lammens M., Deschodt-Lanckman M., and Christophe J. P. (1978) Specific binding of vasoactive intestinal peptide to brain membranes from the guinea pig. Eur. J. Biochem. 90, 147–154.
Robberecht P., Konig W., Deschodt-Lanckman M., De Neef P., and Christophe J. (1979) Specificity of receptors to vasoactive intestinal peptide in guinea pig brain. Life Sci. 25, 879–884.
Rozenboim I. and El Halawani M. E. (1993) Characterization of vasoactive intestinal peptide pituitary membrane receptors in turkey hens during different stages of reproduction. Biol. Reprod. 48, 1129–1134.
Said S. I. and Mutt V. (1970) Polypeptide with broad biological activity: isolation from small intestine. Nature 225, 863–864.
Salomon Y., Londos C., and Rodbell M. (1974) A highly sensitive adenylate cyclase assay. Anal. Biochem. 58, 541–548.
Sherwood N. M., Krueckl S. L., and McRory J. E. (2000) The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr. Rev. 21, 619–670.
Shimizu H., Daly J. W., and Creveling C. R. (1969) A radioisotopic method for measuring the formation of adenosine 3′,5′-cyclic monophosphate in incubated slices of brain. J. Neurochem. 16, 1609–1619.
Staun-Olsen P., Ottesen B., Bartels P. D., Nielsen M. H., Gammeltoft S., and Fahrenkrug J. (1982) Receptors for vasoactive intestinal polypeptide on isolated synaptosomes from rat cerebral cortex. Heterogeneity of binding and desensitization of receptors. J. Neurochem. 39, 1242–1251.
Strange P. G. (1999) G-protein coupled receptors: conformations and states. Biochem. Pharmacol. 58, 1081–1088.
Vaudry D., Gonzales B. J., Basille M., Yon L., Fournier A., and Vaudry H. (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 52, 269–324.
Yoo S.-J., You S., Kim H., Kim S.-C., Choi Y.-J., El Halawani M. E., et al. (2000) Molecular cloning and characterization of alternatively spliced transcripts of the turkey pituitary adenylate cyclase-activating polypeptide. Gen. Comp. Endocrinol. 120, 326–335.
Zawilska J. B., Niewiadomski P., and Nowak J. Z. (2003) Characterization of vasoactive intestinal peptide/pituitary adenylate cyclase-activating polypeptide receptors in chick cerebral cortex. J. Mol. Neurosci. 20, 77–85.
Zawilska J. B., Niewiadomski P., and Nowak J. Z. (2004a) Receptors for vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) in turkey cerebral cortex: characterization by [125I]-VIP binding and effects on cyclic AMP formation. Gen. Comp. Endocrinol. 137, 187–195.
Zawilska J. B., Niewiadomski P., and Nowak J. Z. (2004b) Receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in turkey cerebral cortex. Pol. J. Pharmacol. 56, 203–211.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zawilska, J.B., Dejda, A., Niewiadomski, P. et al. Receptors for VIP and PACAP in guinea pig cerebral cortex. J Mol Neurosci 25, 215–224 (2005). https://doi.org/10.1385/JMN:25:3:215
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:25:3:215