Abstract
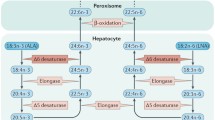
Polyunsaturated fatty acids (PUFA), which comprise 25–30% of the fatty acids in the human brain, are necessary for normal brain development and function. PUFA cannot be synthesized de novo and must be supplied to the brain by the plasma. It is necessary to know the PUFA content and composition of the various plasma lipids and lipoproteins in order to understand how these fatty acids are taken up and metabolized by the brain. Human plasma free fatty acid (FFA) ordinarily contains about 15% linoleic acid (18:2n–6) and 1% arachidonic acid (AA) (20:4n–6). Plasma triglycerides, phospholipids, and cholesterol esters also are rich in linoleic acid, and the phospholipids and cholesterol esters contain about 10% AA. These findings suggest that the brain probably can obtain an adequate supply of n-6 PUFA from either the plasma FFA or lipoproteins. By contrast, the plasma ordinarily contains only one-tenth as much n-3 PUFA, and the amounts range from 1% α-linolenic acid (18:3n–3) in the plasma FFA to 2% docosahexaenoic acid (22:6n–3, DHA) in the plasma phospholipids. The main n-3 PUFA in the brain is DHA. Therefore, if the plasma FFA is the primary source of fatty acid for the brain, much of the DHA must be synthesized in the brain from n-3 PUFA precursors. Alternatively, if the brain requires large amounts of preformed DHA, the phospholipids contained in plasma lipoproteins are the most likely source.
Similar content being viewed by others
References
Bernoud N., Fenart L., Moliere P., Lagarde M., Cecchelli R., and Lecerf J. (1999) Preferential transfer of 2-docosahexaenoyl-1-lysophosphatidylcholine through an in vitro blood-brain barrier over unesterified docosahexaenoic acid. J. Neurochem. 72, 338–345.
Brecher P. and Kuan H. T. (1979) Lipoprotein lipase and acid lipase activity in rabbit brain microvessels. J. Lipid Res. 20, 464–471.
Connor W. E., Lowensohn R., and Hatcher L. (1996) Increased docosahexaenoic acid levels in human newborn infants by administration of sardines and fish oil during pregnancy. Lipids 31, S183-S187.
Conquer J. A. and Holub B. J. (1998) Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J. Lipid Res. 39, 286–292.
DeGeorge J. J., Nariri T., Yamazaki S., Williams W. M., and Rapoport S. I. (1989) Intravenous injection of [1-14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J. Neurosci. Res. 24, 413–423.
Dehouck B., Dehouck M. P., Fruchart J. C., and Cecchelli R. (1994) Upregulation of the low density lipoprotein receptor at the blood: brain barrier: intercommunication between brain capillary endothelial cells and astrocytes. J. Cell Biol. 126, 465–473.
Dehouck B., Fenart L., Dehouck M. P., Pierce A., Torpier G., and Cecchelli R. (1997) A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 138, 877–889.
de Vries H. F., Kuiper J., de Boer A. G., van Verkel T. J., and Breimer D. D. (1993) Characterization of the scavenger receptor on bovine cerebral cerebral endothelial cells in vitro. J. Neurochem. 61, 1813–1821.
Delton-Vanderbroucke I., Grammas P., and Anderson R. E. (1997). Polyunsaturated fatty acid metabolism in retinal and cerebral microvascular endothelial cells. J. Lipid Res. 38, 147–159.
Dhopeshwarkar G. A. and Subramanian C. (1976) Biosynthesis of polyunsaturated fatty acids in the developing brain: I. Metabolic transformations of intracranially administered 1-14C linolenic acid. Lipids 11, 67–71.
Edelstein C. (1986) General properties of plasma lipoproteins and apolipoproteins, in Biochemistry and Biology of Plasma Lipoproteins (Scanu, A. M. and Spector, A. A., eds.), Marcel Dekker, New York, NY, pp. 495–505.
Goodman D. S. and Shiratori T. (1964) Fatty acid composition of human plasma lipoprotein fractions. J. Lipid Res. 5, 307–313.
Hansen J.-B., Grimsgaard S., Nilsen H., Nordoy A., and Bonaa K. H. (1998) Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on fatty acid absorption, incorporation into serum phospholipids and postprandial triglyceridemia. Lipids 33, 131–138.
Innis S. M., Auestad N., and Siegman J. S. (1996) Blood lipid docosahexaenoic and arachidonic acid in term gestation infants fed formulas with high docosahexaenoic acid, low eicosapentaenoic acid fish oil. Lipids 31, 617–625.
Lagarde M., Bernoud N., Brossard N., Lemaitre-Delaunay D., Thies F., Croset M., and Lecerf J. (2001) Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 16 (2–3),
Lands W. E. M., Libelt B., Morris A., Kramer N. C., Prewitt T. E., Bowen P., et al. (1992) Maintenance of lower proportions of (n-6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n-3) fatty acids. Biochim. Biophys. Acta. 1180, 147–162.
Lucarelli M., Gennarelli M., Cardelli P., Novelli G., Scarpa S., Dallapiccola B., and Strom R. (1997) Expression of receptors for native and chemically modified low-density lipoproteins in brain microvessels. FEBS Lett. 401, 53–58.
Martin D. D., Robbins M. E. C., Spector A. A., Wen B.-C., and Hussey, D. H. (1996) The fatty acid composition of human gliomas differs from that found in nonmalignant brain tissue. Lipids 31, 1283–1288.
Martin-Nizard F., Meresse S., Cecchelli R., Fruchart J. C., and Delbart C. (1989) Interactions of high-density lipoprotein 3 with brain capillary endothelial cells. Biochim. Biophys. Acta. 1005, 201–208.
Moore S. A., Yoder E., and Spector A. A. (1990) Role of the blood-brain barrier in the formation of long-chain ω-3 and ω-6 fatty acids from essential fatty acid precursors. J. Neurochem. 55, 391–402.
Moore S. A., Yoder E., Murphy S., Dutton G. R., and Spector A. A. (1991) Astrocytes, not neurons, produce docosahexaenoic acid (22;6ω-3) and arachidonic acid (20;4ω-6). J. Neurochem. 56, 518–524.
Morrisett J. D., Pownall H. J., Jackson R. L., Segura R., Gotto A. M., Jr., and Taunton O. D. (1976). Effects of polyunsaturated and saturated fat diets on the chemical composition and thermotropic properties of human plasma lipoproteins, in Polyunsaturated Fatty Acids (Kunau, W. H. and Holman, R. T., eds.), American Oil Chemists’ Society, Champaign, IL, pp. 139–161.
Nariri T., DeGeorge J. J., Lamour Y., and Rapoport, S. I. (1991) In vivo incorporation of [1-14C]arachidonate in awake rats, with and without cholinergic stimulation, following unilateral lesioning of nucleus basalis magnocellularis. Brain Res. 559, 1–9.
Nariri T., Greig N. H., DeGeorge J. J., Genka S., and Rapoport, S. I. (1993) Intravenously injected radiolabeled fatty acids image brain tumor phospholipids in vivo: differential uptakes of palmitate, arachidonate and docosahexaenoate. Clin. Exp. Metastasis. 11, 141–149.
Nouvelot A., Delbart C., and Bourre, J. M. (1986) Hepatic metabolism of alpha-linolenic acid in suckling rats, and its possible importance in polyunsaturated fatt acid uptake by the brain. Ann. Nutr. Metab. 30, 316–323.
Pace-Asciak C. (1989) One-step rapid extractive methylation of plasma nonesterified fatty acids for gas chromatographic analysis. J. Lipid Res. 30, 451–454.
Pawlosky R. J., Ward G., and Salem N. Jr. (1996) Essential fatty acid uptake and metabolism in the developing rodent brain. Lipids 31, S103-S107.
Purdon D., Arai T., and Rapoport S. I. (1997) No evidence for direct incorporation of esterified palmitate from plasma into brain lipids of awake adult rats. J. Lipid Res. 38, 526–530.
Rogiers V. (1981) Long chain nonesterified fatty acid patterns in plasma of healthy children and young adults in relation to age and sex. J. Lipid Res. 22, 1–16.
Scott B. L. and Bazan N. G. (1989) Membrane docosahexaenoic acid is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci. USA 86, 2903–2907.
Seidelin K. N. (1995) Fatty acid composition of adipose tissue in humans. Implications for the dietary fat-serum cholesterol-CHD issue. Prog. Lipid Res. 34, 199–217.
Sheaff Greiner R. C., Zhang Q., Goodman K. J., Giussani D. A., Nathanielsz P. W., and Brenna J. T. (1996) Linoleate, α-linolenate, and docosahexaenoate recycling into saturated and monounsaturated fatty acids is a major pathway in pregnant or lactating adults and fetal or infant rhesus monkeys. J. Lipid Res. 37, 2675–2686.
Spector A. A. (1986) Plasma albumin as a lipoprotein, in Biochemistry and Biology of Plasma Lipoproteins (Scanu, A. M. and Spector, A. A., eds.), Marcel Dekker, New York, NY, pp. 247–279.
Spector A. A., Moore S. A., and Yorek M. A. (1999) Docosahexaenoic acid metabolism and function in the central nervous system: studies with cell culture model systems, in Essential Fatty Acids and Eicosanoids: Invited Papers from the Fourth International Symposium (Riemersma, R., Kelly, R. W. and Wilson, R., eds.), AOCS Press, Champaign, IL, pp. 16–18.
Spector A. A. (1999) Essentiality of fatty acids. Lipids 34, S1-S3.
Washizaki K., Smith Q. R., Rapoport S. I., and Purdon A. D. (1994) Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in anesthetized rat. J. Neurochem. 63, 727–736.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spector, A.A. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci 16, 159–165 (2001). https://doi.org/10.1385/JMN:16:2-3:159
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:16:2-3:159