Abstract
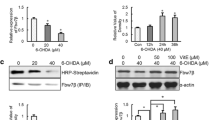
Mutations in familial Parkinson’s disease (PD) have been associated with the failure of protein degradation through the ubiquitin-proteasome system (UPS). Impairment of proteasome function has also been suggested to play a role in the pathogenesis of sporadic PD. We examined the proteasome activity in PC12 cells treated with 6-hydroxydopamine (6-OHDA), the dopamine synthetic derivate used in models of PD. We found that 6-OHDA treatment increased protein oxidation, as indicated by carbonyl group accumulation, and increased caspase-3 activity. In addition, there was an increase in trypsin-, chymotrypsin-, and postacidic-like proteasome activities in cells treated with 10–100 µM 6-OHDA, whereas higher doses caused a marked decline. 6-OHDA exposure also increased mRNA expression of the 19S regulatory subunit in a dose-dependent manner, whereas the expression of 20S- and 11S-subunit mRNAs did not change. Administration of the antioxidant N-acetylcysteine to 6-OHDA-treated cells prevented the alteration in proteasome functions. Moreover, reduction in cell viability owing to administration of proteasome inhibitor MG132 or lactacystin was partially prevented by the endogenous antioxidant-reduced glutathione. In conclusion, our data indicate that mild oxidative stress elevates proteasome activity in response to increase in protein damage. Severe oxidative insult might cause UPS failure, which leads to protein aggregation and cell death. Moreover, in the case of UPS inhibition or failure, the blockade of physiological reactive oxygen species production during normal aerobic metabolism is enough to ameliorate cell viability. Control of protein clearance by potent, brain-penetrating antioxidants might act to slow down the progression of PD.
Similar content being viewed by others
References
Alam Z. I., Daniel S. E., Lees A. J., Marsden D. C., Jenner P., and Halliwell B. (1997a) A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J. Neurochem. 69, 1326–1329.
Alam Z. I., Jenner A., Daniel S. E., Lees A. J., Cairns N., Marsden C. D., et al. (1997b) Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 69, 1196–1203.
Alves-Rodrigues A., Gregori L., and Figueiredo-Pereira M. E. (1998) Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 21, 516–520.
Andrew R., Watson D. G., Best S. A., Midgley J. M., Wenlong H., and Petty R. K. (1993) The determination of hydroxydopamines and other trace amines in the urine of parkinsonian patients and normal controls. Neurochem. Res. 18, 1175–1177.
Barzilai A., Zilkha-Falb R., Daily D., Stern N., Offen D., Ziv I., et al. (2000) The molecular mechanism of dopamine-induced apoptosis: identification and characterization of genes that mediate dopamine toxicity. J. Neural Transm. Suppl. 60, 59–76.
Beal M. F. (1992) Mechanisms of excitotoxicity in neurologic diseases. FASEB J. 6, 3338–3344.
Bence N. F., Sampat R. M., and Kopito R. R. (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555.
Bennett M. C., Bishop J. F., Leng Y., Chock P. B., Chase T. N., and Mouradian M. M. (1999) Degradation of alpha-synuclein by proteasome. J. Biol. Chem. 274, 33,855–33,858.
Borenfreund J. A. and Puerner A. (1984) A simple quantitative procedure using monolayer culture for cytotoxicity assay. J. Tissue Culture Methods 9, 7–9.
Bulteau A. L., Petropoulos I., and Friguet B. (2000) Age-related alterations of proteasome structure and function in aging epidermis. Exp. Gerontol. 35, 767–777.
Chomczynski P. and Sacchi N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159.
Conway K. A., Harper J. D., and Lansbury P. T. (1998) Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med. 4, 1318–1320.
Coux O., Tanaka K., and Goldberg A. L. (1996) Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65, 801–847.
Coyle J. T. and Puttfarcken P. (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262, 689–695.
Davies K. J. (1987) Protein damage and degradation by oxygen radicals: I. General aspects. J. Biol. Chem. 262, 9895–9901.
Davies K. J. A. (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie. 83, 301–310.
Dexter D. T., Holley A. E., Flitter W. D., Slater T. F., Wells F. R., Daniel S. E., et al. (1994) Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov. Disord. 9, 92–97.
Dexter D. T., Wells F. R., Agid F., Agid Y., Lees A. J., Jenner P., and Marsden C. D. (1987) Increased nigral iron content in postmortem parkinsonian brain. Lancet 2, 1219–1220.
Ding Q. and Keller J. N. (2001) Proteasomes and proteasome inhibition in the central nervous system. Free Radic. Biol. Med. 31, 574–584.
Elkon H., Melamed E., and Offen D. (2001) 6-Hydroxy-dopamine increases ubiquitin-conjugates and protein degradation: implications for the pathogenesis of Parkinson’s disease. Cell. Mol. Neurobiol. 21, 771–781.
Figueiredo-Pereira M. E. and Cohen G. (1999) The ubiquitin/proteasome pathway: friend or foe in zinc-, cadmium-, and H2O2-induced neuronal oxidative stress. Mol. Biol. Rep. 26, 65–69.
Floor E. and Wetzel M. G. (1998) Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 70, 268–275.
Forno L. S. (1996) Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 55, 259–272.
Friguet B. and Szweda L. I. (1997) Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 405, 21–25.
Giasson B. I., Duda J. E., Murray I. V., Chen Q., Souza J. M., Hurtig H. I., et al. (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290, 985–989.
Gibb W. R. and Lees A. J. (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 51, 745–752.
Gilgun-Sherki Y., Rosenbaum Z., Melamed E., and Offen D. (2002) Antioxidant therapy in acute central nervous system injury: current state. Pharmacol. Rev. 54, 271–284.
Glickman M. H. and Ciechanover A. (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82, 373–428.
Glockzin, S. von Knethen A., Scheffner M., and Brune B. (1999) Activation of the cell death program by nitric oxide involves inhibition of the proteasome. J. Biol. Chem. 274, 19,581–19,586.
Goedert M. (2001) Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2, 492–501.
Graham D. G. (1978) Oxidative pathways for catecholamine in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 14, 633–643.
Grune, T., Blasig I. E., Sitte N., Roloff B., Haseloff R., and Davies K. J. (1998) Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteasome. J. Biol. Chem. 273, 10,857–10,862.
Halliwell B. and Jenner P. (1998) Impaired clearance of oxidised proteins in neurodegenerative diseases. Lancet 351, 1510.
Hasegawa E., Takeshige K., Oishi T., Murai Y., and Minakami S. (1990) 1-Methyl-4-phenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem. Biophys. Res. Commun. 170, 1049–1055.
Hayashi T. and Goto S. (1998) Age-related changes in the 20S and 26S proteasome activities in the liver of male F344 rats. Mech. Ageing Dev. 102, 55–66.
Jellinger K., Linert L., Kienzl E., Herlinger E., and Youdim M. B. (1995) Chemical evidence for 6-hydroxydopamine to be an endogenous toxic factor in the pathogenesis of Parkinson’s disease. J. Neural Transm. Suppl. 46, 297–314.
Jenner P. and Olanow C. W. (1998) Understanding cell death in Parkinson’s disease. Ann. Neurol. 44, S72-S84.
Keller J. N., Hanni K. B., and Markesbery W. R. (2000a) Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech. Ageing Dev. 113, 61–70.
Keller J. N., Huang F. F., Dimayuga E. R., and Maragos W. F. (2000b) Dopamine induces proteasome inhibition in neural PC12 cell line. Free Radic. Biol. Med. 29, 1037–1042.
Keller J. N., Huang F. F., and Markesbery W. R. (2000c) Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience 98, 149–156.
Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., et al. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608.
Kruger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S., et al. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 18, 106–108.
Leroy E., Boyer R., Auburger G., Leube B., Ulm G., Mezey E., et al. (1998) The ubiquitin pathway in Parkinson’s disease. Nature 395, 451, 452.
Lewy F. H. (1912) Paralysis agitans. In: Pathologische Anatomie, Lewandowsky, M., ed., Springer, Berlin, pp. 920–933.
Lowe J., McDermott H., Landon M., Mayer R. J., and Wilkinson K. D. (1990) Ubiquitin carboxyl-terminal hydrolase (PGP 9.5) is selectively present in ubiquitinated inclusion bodies characteristic of human neurodegenerative diseases. J. Pathol. 161, 153–160.
McNaught K. S. and Jenner P. (2001) Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci. Lett. 297, 191–194.
McNaught K. S., Belizaire R., Isacson O., Jenner P., and Olanow C. W. (2003) Altered proteasomal function in sporadic Parkinson’s disease. Exp. Neurol. 179, 38–46.
McNaught K. S., Belizaire R., Jenner P., Olanow C. W., and Isacson O. (2002) Selective loss of 20S proteasome alpha-subunits in the substantia nigra pars compacta in Parkinson’s disease. Neurosci. Lett. 326, 155–158.
McNaught K. S., Olanow C. W., Halliwell B., Isacson O., and Jenner P. (2001) Failure of the ubiquitin-proteasome system in Parkinson’s disease. Nat. Rev. Neurosci. 2, 589–594.
Mizuno Y., Ikebe S., Hattori N., Nakagawa-Hattori Y., Mochizuki H., Tanaka M., and Ozawa T. (1995) Role of mitochondria in the etiology and pathogenesis of Parkinson’s disease. Biochim. Biophys. Acta 1271, 265–274.
Mizuno Y., Ohta S., Tanaka M., Takamiya S., Suzuki K., Sato T., et al. (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem. Biophys. Res. Commun. 163, 1450–1455.
Narhi L., Wood S. J., Steavenson S., Jiang Y., Wu G. M., Anafi D., et al. (1999) Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J. Biol. Chem. 274, 9843–9846.
Offen D., Gorodin S., Melamed E., Hanania J., and Malik Z. (1999) Dopamine-melanin is actively phagocytized by PC12 cells and cerebellar granular cells: possible implications for the etiology of Parkinson’s disease. Neurosci. Lett. 260, 101–104.
Offen D., Ziv I., Barzilai A., Gorodin S., Glater E., Hochman A., and Melamed E. (1997) Dopamine-melanin induces apoptosis in PC12 cells; possible implications for the etiology of Parkinson’s disease. Neurochem. Int. 31, 207–216.
Offen D., Ziv I., Sternin H., Melamed E., and Hochman A. (1996) Prevention of dopamine-induced cell death by thiol antioxidants: possible implications for treatment of Parkinson’s disease. Exp. Neurol. 141, 32–39.
Parkinson J. (1817) An Essay on the Shaking Palsy, Sherwood, Neely and Jones, London.
Perry T. L., Godin D. V., and Hansen S. (1982) Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci. Lett. 33, 305–310.
Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047.
Reinheckel T., Sitte N., Ullrich O., Kuckelkorn U., Davies K. J., and Grune T. (1998) Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem. J. 335, 637–642.
Sambrook J., Fritsch E. F., and Maniatis T. (1989) Molecular Cloning, a Laboratory Mannual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
Schapira A. H., Cooper J. M., Dexter D., Clark J. B., Jenner P., and Marsden C. D. (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J. Neurochem. 54, 823–827.
Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., et al. (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 25, 302–305.
Shimura H., Schlossmacher M. G., Hattori N., Frosch M. P., Trockenbacher A., Schneider R., et al. (2001) Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson’s disease. Science 293, 263–269.
Shringarpure R., Grune T., Sitte N., and Davies K. J. (2000) 4-Hydroxynonenal-modified amyloid-beta peptide inhibits the proteasome: possible importance in Alzheimer’s disease. Cell. Mol. Life Sci. 57, 1802–1809.
Sian J., Dexter D. T., Lees A. J., Daniel S., Jenner P., and Marsden C. D. (1994) Glutathione-related enzymes in brain in Parkinson’s disease. Ann. Neurol. 36, 356–361.
Simantov R., Blinder E., Ratovitski T., Tauber M., Gabbay M., and Porat S. (1996) Dopamine-induced apoptosis in human neuronal cells: inhibition by nucleic acids antisense to the dopamine transporter. Neuroscience 74, 39–50.
Sofic E., Lange K. W., Jellinger K., and Riederer P. (1992) Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci. Lett. 142, 128–130.
Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M., and Goedert M. (1998) Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473.
Stefanis L., Larsen K. E., Rideout H. J., Sulzer D., and Greene L. A. (2001) Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J. Neurosci. 21, 9549–9560.
Strack P. R., Waxman L., and Fagan J. M. (1996) Activation of the multicatalytic endopeptidase by oxidants. Effects on enzyme structure. Biochemistry 35, 7142–7149.
Swerdlow R. H., Parks J. K., Miller S. W., Tuttle J. B., Trimmer P. A., Sheehan J. P., et al. (1996) Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann. Neurol. 40, 663–671.
Yoritaka A., Hattori N., Uchida K., Tanaka M., Stadtman E. R., and Mizuno Y. (1996) Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. U.S.A. 93, 2696–2701.
Youdim M. B., Ben-Shachar D., and Riederer P. (1989) Is Parkinson’s disease a progressive siderosis of substantia nigra resulting in iron and melanin induced neurodegeneration? Acta Neurol. Scand. Suppl. 126, 47–54.
Ziv I., Melamed E., Nardi N., Luria D., Achiron A., Offen D., and Barzilai A. (1994) Dopamine induces apoptosis-like cell death in cultured chick sympathetic neurons, a possible novel pathogenetic mechanism in Parkinson’s disease. Neurosci. Lett. 170, 136–140.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elkon, H., Melamed, E. & Offen, D. Oxidative stress, induced by 6-hydroxydopamine, reduces proteasome activities in PC12 cells. J Mol Neurosci 24, 387–400 (2004). https://doi.org/10.1385/JMN:24:3:387
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:24:3:387