Abstract
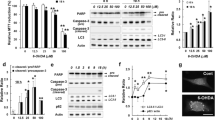
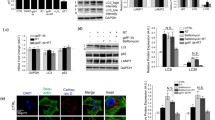
Parkinson’s disease (PD) is the most common movement disorder disease, and its pathological feature is the degenerative loss of dopaminergic neurons in the substantia nigra compacta (SNc). In this study, we investigated whether distinct stress conditions target F-box protein Fbw7β via converging mechanisms. Our results showed that the 6-hyroxydopamine (6-OHDA), which causes PD in animals’ models, led to decreased stability of Fbw7β in DA neuronal SN4741 cells. Further experiments suggested that oxidized Fbw7β bound to heat-shock cognate protein 70 kDa, the key regulator for chaperone-mediated autophagy (CMA), at a higher affinity. Oxidative stress also increased the level of lysosomal-associated membrane protein 2A (LAMP2A), the rate-limiting receptor for CMA substrate flux, and stimulated CMA activity. These changes resulted in accelerated degradation of Fbw7β. 6-OHDA induced Fbw7β oxidation and increased LAMP2A in the SNc region of the mouse models. Consistently, the levels of oxidized Fbw7β were higher in postmortem PD brains compared with the controls. These findings for the first time revealed the specific mechanism of ubiquitin ligases, oxidative stress, and CMA-mediated protein degradation, to provide a new theoretical basis for further clarifying the mechanism of PD.





Similar content being viewed by others
References
Kalia LV, Lang AE (2016) Parkinson disease in 2015: evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol 12(2):65–66. doi:10.1038/nrneurol.2015.249
Rizek P, Kumar N, Jog MS (2016) An update on the diagnosis and treatment of Parkinson disease. CMAJ De l'Association Medicale Canadienne 188(16):1157–1165. doi:10.1503/cmaj.151179
Gratwicke J, Jahanshahi M, Foltynie T (2015) Parkinson’s disease dementia: a neural networks perspective. Brain 138(Pt 6):1454–1476. doi:10.1093/brain/awv104
Wang B, Abraham N, Gao G, Yang Q (2016) Dysregulation of autophagy and mitochondrial function in Parkinson’s disease. Transl Neurodegener 5:19. doi:10.1186/s40035-016-0065-1
Kenific CM, Debnath J (2015) Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol 25(1):37–45. doi:10.1016/j.tcb.2014.09.001
Lapierre LR, Kumsta C, Sandri M, Ballabio A, Hansen M (2015) Transcriptional and epigenetic regulation of autophagy in aging. Autophagy 11(6):867–880. doi:10.1080/15548627.2015.1034410
Joven J, Guirro M, Marine-Casado R, Rodriguez-Gallego E, Menendez JA (2014) Autophagy is an inflammation-related defensive mechanism against disease. Adv Exp Med Biol 824:43–59. doi:10.1007/978-3-319-07320-0_6
Orogo AM, Gustafsson AB (2015) Therapeutic targeting of autophagy: potential and concerns in treating cardiovascular disease. Circ Res 116(3):489–503. doi:10.1161/CIRCRESAHA.116.303791
Xilouri M, Stefanis L (2010) Autophagy in the central nervous system: implications for neurodegenerative disorders. CNS Neurol Disord Drug Targets 9(6):701–719
Hu Z, Yang B, Mo X, Xiao H (2015) Mechanism and regulation of autophagy and its role in neuronal diseases. Mol Neurobiol 52(3):1190–1209. doi:10.1007/s12035-014-8921-4
Zare-Shahabadi A, Masliah E, Johnson GV, Rezaei N (2015) Autophagy in Alzheimer’s disease. Rev Neurosci 26(4):385–395. doi:10.1515/revneuro-2014-0076
Trinh J, Farrer M (2013) Advances in the genetics of Parkinson disease. Nat Rev Neurol 9(8):445–454. doi:10.1038/nrneurol.2013.132
Martin DD, Ladha S, Ehrnhoefer DE, Hayden MR (2015) Autophagy in Huntington disease and huntingtin in autophagy. Trends Neurosci 38(1):26–35. doi:10.1016/j.tins.2014.09.003
Lee JK, Shin JH, Lee JE, Choi EJ (2015) Role of autophagy in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta 1852(11):2517–2524. doi:10.1016/j.bbadis.2015.08.005
Wei K, Wang P, Miao CY (2012) A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci Ther 18(11):879–886. doi:10.1111/cns.12005
Lipinski MM, Wu J, Faden AI, Sarkar C (2015) Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal 23(6):565–577. doi:10.1089/ars.2015.6306
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441(7095):885–889. doi:10.1038/nature04724
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884. doi:10.1038/nature04723
Kaushik S, Cuervo AM (2012) Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol 22(8):407–417. doi:10.1016/j.tcb.2012.05.006
Kabuta T, Furuta A, Aoki S, Furuta K, Wada K (2008) Aberrant interaction between Parkinson disease-associated mutant UCH-L1 and the lysosomal receptor for chaperone-mediated autophagy. J Biol Chem 283(35):23731–23738. doi:10.1074/jbc.M801918200
Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS et al (2013) Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci 16(4):394–406. doi:10.1038/nn.3350
Bejarano E, Cuervo AM (2010) Chaperone-mediated autophagy. Proc Am Thorac Soc 7(1):29–39. doi:10.1513/pats.200909-102JS
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson’s disease. Front Neuroanat 9:91. doi:10.3389/fnana.2015.00091
Chaturvedi RK, Flint Beal M (2013) Mitochondrial diseases of the brain. Free Radic Biol Med 63:1–29. doi:10.1016/j.freeradbiomed.2013.03.018
Ekholm-Reed S, Goldberg MS, Schlossmacher MG, Reed SI (2013) Parkin-dependent degradation of the F-box protein Fbw7beta promotes neuronal survival in response to oxidative stress by stabilizing Mcl-1. Mol Cell Biol 33(18):3627–3643. doi:10.1128/MCB.00535-13
Cai Z, Zeng W, Tao K, E Zhen, Wang B, Yang Q (2015) Chaperone-mediated autophagy: roles in neuroprotection. Neurosci Bull 31 (4):452–458. doi:10.1007/s12264-015-1540-x
Johansson S, Lee IH, Olson L, Spenger C (2005) Olfactory ensheathing glial co-grafts improve functional recovery in rats with 6-OHDA lesions. Brain 128(Pt 12):2961–2976. doi:10.1093/brain/awh644
Magraoui FE, Reidick C, Meyer HE, Platta HW (2015) Autophagy-related deubiquitinating enzymes involved in health and disease. Cell 4(4):596–621. doi:10.3390/cells4040596
Chen S (2014) Chinese guidelines for the diagnosis criteria and treatment of Parkinson’s disease (3rd edition). Chin J Neurol 47(6):428–433. doi:10.3760/cma.j.issn.1006-7876.2014.06
Gao L, She H, Li W, Zeng J, Zhu J, Jones DP, Mao Z, Gao G et al (2014) Oxidation of survival factor MEF2D in neuronal death and Parkinson’s disease. Antioxid Redox Signal 20(18):2936–2948. doi:10.1089/ars.2013.5399
Song J, Kim J (2016) Degeneration of dopaminergic neurons due to metabolic alterations and Parkinson’s disease. Front Aging Neurosci 8:65. doi:10.3389/fnagi.2016.00065
Subramaniam SR, Chesselet MF (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 106-107:17–32. doi:10.1016/j.pneurobio.2013.04.004
Saha T (2012) LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy 8(11):1643–1656. doi:10.4161/auto.21654
Zhang S, Gui XH, Huang LP, Deng MZ, Fang RM, Ke XH, He YP, Li L et al (2016) Neuroprotective effects of beta-asarone against 6-hydroxy dopamine-induced parkinsonism via JNK/Bcl-2/Beclin-1 pathway. Mol Neurobiol 53(1):83–94. doi:10.1007/s12035-014-8950-z
Huang L, Xue Y, Feng D, Yang R, Nie T, Zhu G, Tao K, Gao G et al (2017) Blockade of RyRs in the ER attenuates 6-OHDA-induced calcium overload, cellular hypo-excitability and apoptosis in dopaminergic neurons. Front Cell Neurosci 11:52. doi:10.3389/fncel.2017.00052
Acknowledgements
The authors thank Ms. Lin Zhang for her English proof reading. This project was supported by grant from the National Natural Science Foundation of China (81471377).
Author information
Authors and Affiliations
Contributions
Xiufeng Wang, acquisition of data, literature research, and manuscript preparation; Heng Zhai, acquisition of data; Fang Wang, study concept and design, critical revision of the manuscript for important intellectual content, and study supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplementary Fig. S1
The overexpression of Fbw7β was confirmed by western blot. SN4741 cells were inoculated into six-well plate to culture till 80% fusion and the pGCSIL vector encoding the mouse Fbw-7β and control was transfected into SN4741 cells (JPEG 62 kb)
Supplementary Fig. S2
Motor activity examination after 6-OHDA lesioning. Amphetamine (2.5 mg/kg)-induced rotations were counted after 6-OHDA lesioning, as described in the supplementary methods. a Time course of rotational behavior. b Total number of rotations in 60 min. Values shown are means ± SD of three experiments (total nine mice in each group). * P < 0.05 (JPEG 181 kb)
Supplementary Fig. S3
Levels of Fbw7β in the SNc region of 6-OHDA-treated mice and PD patients. a Lysates prepared from 6-OHDA-treated mice brains were analyzed for Fbw7β by western blot (* P < 0.05). b Fbw7β levels in the brain of human PD patients analyzed by western blot (JPEG 216 kb)
Supplementary Fig. S4
The oxidization level of HMGB1 in PD patients and control. The lysates were prepared from the striata of postmortem PD patients and controls (JPEG 56 kb)
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Zhai, H. & Wang, F. 6-OHDA Induces Oxidation of F-box Protein Fbw7β by Chaperone-Mediated Autophagy in Parkinson’s Model. Mol Neurobiol 55, 4825–4833 (2018). https://doi.org/10.1007/s12035-017-0686-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0686-0