Abstract
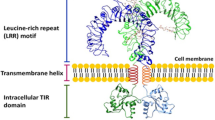
Toll-like receptors (TLRs) play a crucial role in the innate immune system as a first line of defense against pathogens. TLR activation in phagocytes produces pro-inflammatory cytokines and chemokines that contribute directly to elimination of infectious agents and activation of adaptive immune responses. However, a sustained inflammatory response can result in tissue damage and generalized sepsis. This review summarizes the complex and sometimes conflicting links of TLR signaling with two important regulators of immune cells functions: phosphoinositide 3-kinases (PI3Ks) and small GTPases of the Rho family. A unified model of hierarchical organization of these signaling participants is still premature, given that the tools for delineating how control of TLR-mediated pathways is achieved are just emerging. Critical progress in our understanding of spatial-temporal propagation of TLR signaling will certainly be provided in the near future by pharmacological targeting of PI3Ks using recently characterized, second-generation PI3K inhibitors in combination with gene-targeting strategies for PI3K subunits and Rho GTPases targeted to the murine myeloid compartment.
Similar content being viewed by others
References
Lemaitre B, Nicolas E, Michaut L, Reichhart, JM, Hoffmann JA: The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996; 86: 973–983.
Medzhitov R, Preston-Hurlburt P, Janeway CA, Jr: A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997; 388: 394–397.
Poltorak A, He, X, Smirnova I, et al: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998; 282: 2085–2088.
Takeda K, Akira S: Toll-like receptors in innate immunity. Int Immunol 2005; 17: 1–14.
Beutler B: Inferences questions and possibilities in Toll-like receptor signalling. Nature 2004; 430: 257–263.
Akira S, Takeda K: Toll-like receptor signalling. Nat Rev Immunol 2004; 4: 499–511.
Rehli M: On mice and men: species variations of Toll-like receptor expression. Trends Immunol 2002; 23: 375–378.
Dunne A, O'Neill LA: The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2003; 2003: 3.
Yamamoto M, Takeda K, Akira S: TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol 2004; 40: 861–868.
Takeuchi O, Kawai T, Muhlradt PF, et al: Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 2001; 13: 933–940.
Takeuchi O, Sato S, Horiuchi T, et al: Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 2002; 169: 10–14.
Kawai T, Akira S: Pathogen recognition, with Toll-like receptors. Curr Opin Immunol 2005; 17: 338–344.
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ: TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001; 412: 346–351.
Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al: Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 2001; 413: 78–83.
Yamamoto M, Sato, S, Hemmi H, et al: Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 2002; 420: 324–329.
Hoebe, K, Du X, Georgel P, et al: Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 2003; 424: 743–748.
Yamamoto M, Sato S, Hemmi H, et al: Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003; 301: 640–643.
Fitzgerald KA, Rowe DC, Barnes BJ, et al: LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 2003; 198: 1043–1055.
Oshiumi H, Sasai M, Shida K, Fujita T, Matsumoto M, Seya T: TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta. J Biol Chem 2003; 278: 49751–49762.
Yamamoto M, Sato S, Hemmi H, et al: TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 2003; 4: 1144–1150.
Deane JA, Fruman DA: Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol 2004; 22: 563–598.
Fruman DA, Cantley LC: Phosphoinositide 3-kinase in immunological systems. Semin Immunol 2002; 14: 7–18.
Koyasu S: The role of PI3K in immune cells. Nat Immunol 2003; 4: 313–319.
Fukao T, Koyasu S: PI3K and negative regulation of TLR signaling. Trends Immunol 2003; 24: 358–363.
Liew FY, Xu D, Brint EK, O'Neill LA: Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 2005; 5: 446–458.
Okugawa S, Ota Y, Kitazawa T, et al: Janus kinase 2 is involved in lipopolysaccharide-induced activation of macrophages. Am J Physiol Cell Physiol 2003; 285: C399–408.
Arbibe L, Mira JP, Teusch N et al: Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol 2000; 1: 533–540.
Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M: Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol 2003; 33: 597–605.
Monick MM, Robeff PK, Butler NS, et al: Phosphatidylinositol 3-kinase activity negatively regulates stability of cyclooxygenase 2 mRNA. J Biol Chem 2002; 277: 32992–33000.
Diaz-Guerra MJ, Castrillo A, Martin-Sanz P, Bosca L: Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J Immunol 1999; 162: 6184–6190.
Park YC, Lee CH, Kang HS, Chung HT, Kim HD: Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase, enhances LPS-induced NO production from murine peritoneal macrophages. Biochem Biophys Res Commun 1997; 240: 692–696.
Weinstein SL, Finn AJ, Dave SH, et al: Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-beta. J Leukoc Biol 2000; 67: 405–414.
Aksoy E, Vanden Berghe W, Detienne S, et al: Inhibition of phosphoinositide 3-kinase enhances TRIF-dependent NF-kappa B activation and IFN-beta synthesis downstream of Toll-like receptor 3 and 4. Eur J Immunol 2005; 35: 2200–2209.
Jiang Z, Mak TW, Sen G, Li X: Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci USA 2004; 101: 3533–3538.
Sato S, Sugiyama M, Yamamoto M, et al: Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol 2003; 171: 4304–4310.
Guha M, Mackman N: The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem 2002; 277: 32124–32132.
Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N: PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol 2004; 24: 1963–1969.
Banchereau J, Briere F, Caux C, et al: Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18: 767–811.
Fukao T, Tanabe M, Terauchi Y, et al: PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol 2002; 3: 875–881.
Fang H, Pengal RA, Cao X, et al: Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J Immunol 2004; 173: 360–366.
Cao X, Wei G, Fang H, et al: The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J Immunol 2005; 174: 4851–4857.
Strassheim D, Kim JY, Park JS, Mitra S, Abraham E: Involvement of SHIP in TLR2-induced neutrophil activation and acute lung injury. J Immunol 2005; 174: 8064–8071.
Pengal RA, Ganesan LP, Wei G, Fang H, Ostrowski MC, Tridandapani S: Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol Immunol 2006; 43: 1557–1564.
Martin M, Rehani K, Jope RS, Michalek SM: Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 2005; 6: 777–784.
Strassheim D, Asehnoune K, Park JS, et al: Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol 2004; 172: 5727–5733.
Hayashi F, Means TK, Luster AD: Toll-like receptors stimulate human neutrophil function. Blood 2003; 102: 2660–2669.
Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C: Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol 2005; 174: 3633–3642.
Ward C, Murray J, Clugston A, Dransfield I, Haslett C, Rossi AG: Interleukin-10 inhibits lipopolysaccharideinduced survival and extracellular signal-regulated kinase activation in human neutrophils Eur J Immunol 2005; 35: 2728–2737.
Park Y, Lee SW, Sung YC: Cutting edge: CpG DNA inhibits dendritic cell apoptosis by up-regulating cellular inhibitor of apoptosis proteins through the phosphatidylinositide-3′-OH kinase pathway. J Immunol 2002; 168: 5–8.
Darieva Z, Lasunskaia EB, Campos MN, Kipnis TL, Da Silva WD: Activation of phosphatidylinositol 3-kinase and c-Jun-N-terminal kinase cascades enhances NF-kappaB-dependent gene transcription in BCG-stimulated macrophages through promotion of p65/p300 binding. J Leukoc Biol 2004; 75: 689–697.
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA: Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001; 413: 732–738.
Sen GC: Viruses and interferons. Annu Rev Microbiol 2001; 55: 255–281.
Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC: Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol 2004; 11: 1060–1067.
Sarkar SN, Smith HL, Rowe TM, Sen GC: Doublestranded RNA signaling by Toll-like receptor 3 requires specific tyrosine residues in its cytoplasmic domain. J Biol Chem 2003; 278: 4393–4396.
Guillot L, Le Goffic R, Bloch S, et al: Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem 2005; 280: 5571–5580.
Walker EH, Pacold ME, Perisic O, et al: Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 2000; 6: 909–919.
Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS: Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase. Chem Biol 2005; 12: 99–107.
Davies SP, Reddy H, Caivano M, Cohen P: Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351: 95–105.
Jacobs MD, Black J, Futer O, et al: Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J Biol Chem 2005; 280: 13728–13734.
Wetzker R, Rommel C: Phosphoinositide 3-kinases as targets for therapeutic intervention. Curr Pharm Des 2004; 10: 1915–1922.
Camps M, Ruckle T, Ji H, et al: Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 2005; 11: 936–943.
Hmama Z, Knutson KL, Herrera-Velit P, Nandan D, Reiner NE: Monocyte adherence induced by lipopolysaccharide involves CD14, LFA-1, and cytohesin-1. Regulation by Rho and phosphatidylinositol 3-kinase. J Biol Chem 1999; 274: 1050–1057.
Barber DF, Bartolome A, Hernandez C, et al: PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 2005; 11: 933–935.
Bilancio A, Okkenhaug K, Camps M, et al.: Key role of the p110{delta} isoform of PI3K in B cell antigen and IL4 receptor signalling—comparative analysis of genetic and pharmacological interference with p110{delta} function in B cells. Blood 2006; 107: 642–650.
Okkenhaug K, Bilancio A, Farjot G, et al.: Impaired B and T cell antigen receptor signaling in p110delta PI3-kinase mutant mice. Science 2002; 297: 1031–1034.
Jackson SP, Schoenwaelder SM, Goncalves I, et al.: PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med 2005; 11: 507–514.
Knight ZA, Chiang GG, Alaimo PJ, et al.: Isoform-specific phosphoinositide 3-kinase inhibitors from an arylmorpholine scaffold. Bioorg Med Chem 2004; 12: 4749–4759.
Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC: Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci 2005; 30: 194–204.
Fruman DA, Mauvais-Jarvis F, Pollard DA, et al.: Hypoglycaemia, liver necrosis and perinatal dealth in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat Genet 2000; 26: 379–382.
Munugalavadla V, Borneo J, Ingram DA, Kapur R: p85alpha subunit of class IA PI-3 kinase is crucial for macrophage growth and migration. Blood 2005; 106: 103–109.
Jaffe AB, Hall A: RHO GTPASES: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21: 247–269.
Wennerberg, K, Der CJ: Rho-family GTPases: it's not only Rac and Rho (and I like it). J Cell 2004; 117: 1301–1312.
Bokoch GM: Regulation of innate immunity by Rho GTPases. Trends Cell Biol 2005; 15: 163–171.
Dinauer MC: Regulation of neutrophil function by Rac GTPases. Curr Opin Hematol 2003; 10: 8–15.
Barbieri JT, Riese MJ, Aktories K: Bacterial toxins that modify the actin cytoskeleton. Annu Rev Cell Dev Biol 2002; 18: 315–344.
Boquet P: p21 GTP-binding proteins and bacterial toxins. Int J Med Microbiol 2000; 290: 429–434.
Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R: TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol 2005; 174: 5016–5023.
Equils O, Madak Z, Liu C, Michelsen KS, Bulut Y, Lu D: Rac1 and Toll-IL-1 receptor domain-containing adapter protein mediate Toll-like receptor 4 induction of HIV-long terminal repeat. J Immunol 2004; 172: 7642–7646.
Patel TR, Corbett, SA: Mevastatin suppresses lipopolysaccharide-induced Rac activation in the human monocyte cell line THP-1. Surgery 2003; 134: 306–311.
Woo CH, Kim JH: Rac GTPase activity is essential for lipopolysaccharide signaling to extracellular signalregulated kinase and p38 MAP kinase activation in rat-2 fibroblasts. Mol Cells 2002; 13: 470–475.
Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D: P-Rex 1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol 2005; 15: 1874–1879.
Gupta S, Lee A, Hu C, et al.: Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum Immunol 2003; 64: 389–401.
Tanaka Y, Bi K, Kitamura R, et al.: SWAP-70-like adapter of T cells, and adapter protein that regulates early TCR-intiated signaling in Th2 lineage cells. Immunity 2003; 18: 403–414.
Weiner OD: Rac activation: P-Rex 1—a convergence point for PIP(3) and Gbetagamma? Curr Biol 2002; 12: R429–431.
Welch HC, Coadwell WJ, Ellson CD, et al.: P-Rex 1, a PtdIns(3,4,5)P3-and Gbetagamma-regulated guaninenucleotide exchange factor for Rac. Cell 2002; 108: 809–821.
Welch HC Coadwell WJ, Stephens LR, Hawkins PT: Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett 2003; 546: 93–97.
Jefferies C, Bowie A, Brady G, Cooke EL, Li X, O'Neill LA: Transactivation by the p65 subunit of NF-kappaB in response to interleukin-1 (IL-1) involves MyD88, IL-1 receptor-associated kinase 1, TRAF-6, and Rac1. Mol Cell Biol 2001; 21: 4544–4552
Ehrhardt C, Kardinal C, Wurzer WJ, et al.: Rac1 and PAK1 are upstream of IKK-epsilon and TBK-1 in the viral activation of interferon regulatory factor-3. FEBS Lett 2004; 567: 230–238.
Kuncewicz T, Balakrishnan P, Snuggs MB, Kone BC: Specific association of nitric oxide synthase-2 with Rac isoforms in activated murine macrophages. Am J Physiol Renal Physiol 2001; 281: F326–336.
Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P: Rac2 is critical for neutrophil primary granule exocytosis. Blood 2004; 104: 832–839.
Kim C, Dinauer MC: Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol 2001; 166: 1223–1232.
Roberts AW, Kim C, Zhen L, et al.: Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 1999; 10: 183–196.
Williams DA, Tao W, Yang F, et al.: Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood 2005; 96: 1646–1654.
Yamauchi A, Kim C, Li S, et al.: Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol 2004; 173: 5971–5979.
Glogauer M, Marchal CC, Zhu F, et al.: Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol 2003; 170: 5652–5657
Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M: Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 2004; 104: 3758–3765.
Carstanjen D, Yamauchi A, Koornneef A et al.: Rac2 regulates neutrophil chemotaxis, superoxide production, and myeloid colony formation through multiple distinct effector pathways. J Immunol 2005; 174: 4613–4620.
Koh AL, Sun CX, Zhu F, Glogauer M: The role of Rac1 and Rac2 in bacterial killing. Cell Immunol 2005; 235: 92–97.
Zhang X, Glogauer M, Zhu F, Kim Th, Chiu B, Inman RD: Innate immunity and arthritis: neutrophil Rac and toll-like receptor 4 expression define outcomes in infection-triggered arthritis. Arthritis Rheum 2005; 52: 1297–1304.
Chen LY, Zuraw BL, Liu FT, Huang S, Pan ZK: IL-1 receptor-associated kinase and low molecular weight GTPase RhoA signal molecules are required for bacterial lipopolysaccharide-induced cytokine gene transcription. J Immunol 2002; 169: 3934–3939.
Teusch N, Lombardo E, Eddleston J, Knaus UG: The low molecular weight GTPase RhoA and atypical protein kinase Czeta are required for TLR2-mediated gene transcription. J Immunol, 2004; 173: 507–514.
Chen LY, Doerner A, Lehmann PF, Huang S, Zhong G, Pan ZK: A novel protein kinase C (PKCepsilon) is required for fMet-Leu-Phe-induced activation of NF-kappaB in human peripheral blood monocytes. J Biol Chem 2005; 280: 22497–22501.
Fessler MB, Arndt PG, Frasch SC, et al.: Lipid rafts regulate lipopolysaccharide-induced activation of Cdc42 and inflammatory functions of the human neutrophil. J Biol Chem 2004; 279: 39989–39998.
Gao Y, Dickerson JB, Guo F, Zheng Y: Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 2004; 101: 7618–7623.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1385/IR:34:3:255.
Rights and permissions
About this article
Cite this article
Ruse, M., Knaus, U.G. New players in TLR-mediated innate immunity. Immunol Res 34, 33–48 (2006). https://doi.org/10.1385/IR:34:1:33
Issue Date:
DOI: https://doi.org/10.1385/IR:34:1:33