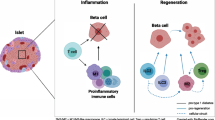
Abstract
Susceptibility and resistance to autoimmune diabetes appear to be regulated at several levels. Whereas the effector functions of autoimmunity are dependent on the antigen-specific responses of autoreactive T cells, the development of tissue-specific autoimmunity appears to be additionally dependent on tissue-specific factors. Thus, the recruitment of T lymphocytes into the vicinity of islet tissue is driven by the local production of chemokines with a pattern unique to islet tissue. The accumulation of lymphocytes triggers events that lead to the local development of new lymphoid tissue, enhancing the presentation of islet-specific antigens to the immune system. In the presence of additional genetic factors allowing persistent immune responses, autoreactivity is permitted to progress until end-stage autoimmune diabetes develops. My laboratory is studying the influence of tissue-specific factors and the genetic regulation of lymphocyte reactivity. An integrated understanding of these effects should lead to a more physiologic approach to prevention and treatment of autoimmune diabetes.
Similar content being viewed by others
References
Lo D, Sprent J: Identity of cells that imprint H-2-restricted T-cell specificity in the thymus. Nature 1986;319:672–675.
Ron Y, Lo D, Sprent J: T cell specificity in tw ice-irradiated F1→parent bone marrow chimeras: fai lure to detect a role for immigrant marrow-derived cells in imprinting intrathymic H-2 restriction. J Immunol 1986;137:1764–1771.
Poirier G, Lo D, Reilly CR, Kaye J: Discrimination between thymic epithelial cells and peripheral antigen presenting cells in the induction of immature T cell differentiation. Immunity 1994;1: 385–391.
Laufer TM, DeKoning J, Markowitz JS Lo D, Glimeher LH: Unopposed positive selection and autoreactivity in mice expressing class 11 MHC only on thymic cortex. Nature 1996;382:81–85.
DeKoning J, DiMolfetto L, Reilly C, Wei Q, Havran W, Lo D: Thymic corticalepithelium is sufficient for the development of mature T cells in RelB-deficient mice. J Immunol 1997;158:2558–2566.
Lo D, Ron Y, Sprent J: Induction of MHC-restricted specificity and tolerance in the thymus. Immunol Res 1986;5:221–232.
Marrack P, Lo D, Brinster R, Palmiter R, Burkly L, FlavellR, Kappler J: The effect of thymus environment on T cell development and tolerance. Cell 1988;53: 627–634.
Burkly LC, Degermann S, Longley J, Hagman J, Brinster RL, Lo D, Flavell RA: Clonal deletion of Vß5+T cells by transgenic 1-E restricted to thymic medullary epithelium. J Immunol 1993;151: 3954–3960.
Degermann S, Surh CD, Glimcher, LH, Sprent J, Lo D: B7 expression on thymic medulary epithelium correlates with epithelium mediated deletion of Vß5+thymocytes. J Immunol 1994;152:3254–3263.
Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D: Expression of RelB is required for the development of thymic medulla and dendritic cells. Nature 1995; 373:531–536.
Naspetti M, Aurrand-Lions M, DeKoning J, Malissen M, Galland F, Lo D, Naquet P: Thymocytes and RelB-dependent medullary epithelial cells provide respectively growth-promoting and organization signals to thymic medullary stromal cells. Eur J Immunol 1997;27:1392–1397.
Lo D, Reilly C, Burkly L, DeKoning J, Laufer T, Glimcher L: Thymic stromal cell specialization and the T cell receptor repertoire. Immunol Res 1997;16:3–14.
Lo D, Reilly CR, Scott B, Liblau R, McDevit HO, Burkly LC: Antigen presenting cells in adoptively transferred and spontaneous diabetes. Eur J Immunol 1993;23: 1693–1698.
Scott B, Liblau R, Degermann S, Marconi LA, Ogata J, Caton AJ, McDevitt HO, Lo D: A role fornon-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity 1994;1 73–82.
Degermann S, Reilly C, Scott B, Ogata L, vonBoehmer H, Lo D: On the various manifestations of spontaneousauto immune diabetes in rodent models. Eur J Immunol 1994;24:3155–3160.
Crowley M, Lo D: Targeted gene knockouts: insights into dendritic cell biology: in Lotze MT, Thomson AW (eds). Dendritic Cells: Biology and Clinical Application. SanDiego, Academic Press, 1999, pp 579–593.
Wu L, D'Amico A, Winkel KD, Suter M, Lo D, Shortman K: RelB is essential for the development of myeloid-related CD8α-but not of lymphoid-related CD8ß+dendritic cells. Immunity 1998;9:839–847.
Gerloni M, Lo D, Zanetti M: DNA immunization in Relß-deficient mice discloses a role for dendritic cells in lgM→IgG1 switch in vivo. Eur J Immunol 1998;28:516–524.
DiMolfetto L, Neal HA, Wu A, Reilly C, Lo D: The density of the class 11 MHC Tcell receptor ligand influences IFN-7/IL-4 ratios in immune responses in vivo. Cell Immunol 1998;183:70–79.
Crowley MT, Reilly CR, Lo D: The influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J Immunol 1999;163:4894–4900.
Lo D, Burkly LC, Widera G Cowing C, Flavell RA, Palmiter RD, Brinster RL: Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell 1988; 53:159–168.
Markmann J, Lo D, Naji A, Palmiter RD, Brinster RL, Heber-Katz E: Antigen presenting function of class II MHC expressing pancreatic beta cells. Nature 1998;336:476–479.
Lo D, Burkly LC, Flavell RA, Palmiter RD, Brinster RL: Tolerence in transgenic mice expressing class II MHC on pancreatic acinar cells. J Exp Med 1989;170:87–104.
Burkly LC, Lo D, Kanagawa O, Brinster RL, Flavell RA: T-celltolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature 1989;342:564–566.
Lo D, Burkly LC, Flavel RA, Palmiter RD, Brinster RL: Antigen presentation in MHC class II transgenic mice: stimulation versus tolerization. Immunol Rev 1990; 117:121–134.
Lo D, Freedman J, Hesse S, Palmiter RD, Brinster RL, Sherman L: Peripheral tolerance to an islet cell specific hemagglutinin transgene affects both CD4+ and CD8+T cells. Eur J Immunol 1992;22:1013–1022.
Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA: CD8+T cell-mediated spontaneous diabetes inneonatal mice. J Immunol 1996;157:978–983.
Lo D, Freedman J, Hesse S, Brinster RL, Sherman L: Peripheral tolerance in transgenic mice: tolerance to class II MHC and non-MHC transgene antigens. Immunol Rev 1991;122: 87–102.
Ho I-C, Lo D, Glimcher LH: C-Maf promotes Th2 and attenuates Thl differentiation by both IL-4 dependent and independent mechanisms. J Exp Med 1998;188: 1859–1866.
Pauza M, Neal H, Hagenbaugh A, Cheroutre H, Lo D: T cell production of an inducible transgene IL-10 provides limited protection from autoimmune diabetes. Diabetes 1999;48:1948–1953.
Pauza M, Smith KM, Neal H, Reilly CR, Lanier LL, Lo D: Transgenic expression of Ly-49A in thymocytes permits positive selection of forbidden clones. J Immunol 2000;164:884–892.
Waldmann H, Cobbold S: How do monoclonal antibodies induce tolerance? A role for infectious tolerance? Annu Rev Immunol 1998;16:619–644.
Wu A, Schulman SJ, Marconi LA, Reilly C, Scott B, Lo D: Protection against diabetes by MHC heterozygosity and reversal by cyclophosphamide. Cell Immunol 1998;184:112–120.
Li L, Crowley MR, Nguyen A, Lo D: Ability of a non-depleting anti-CD4 antibody to inhibit Th2 responses and allergic lung inflamation is independent of co-receptor function. J Immunol 1999;163: 6557–6566.
Xia Y, Pauza M, Feng L, Lo D: RelB regulation of chemokine expression modulates local inflammation. Am J Pathol 1997;151: 375–387.
Xia Y, Chen S, Wang Y, Mackman N, Ku G, Lo D, Feng L: RelB modulation of 1kbαstability as a mechanism of transcription suppression of IL-1α, IL-1ß, and TNFα in fibroblasts. Mol Cell Biol 1999:19: 7688–7696.
Lo D, Feng L, Li L, Carson MJ, Crowley M, Pauza M, Nguyen A, Reilly C: Integrating innate and adaptive immunity in the whole animal. Immunol Rev 1999;169: 225–239.
Li L, Xia Y, Nguyen A, Feng L, Lo D: Th2 inducedeo taxin expression and eosinophilia coexist with Thl responses at the effector stage of lung inflammation. J Immunol 1998;161:3128–3135.
Li L, Xia Y, Nguyen A, Lai YH, Feng L, Mosmann TR, Lo D: Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by lungpithelial cells. J Immunol 1999;162: 2477–2487.
Tanabe S, Lu Z, Luo Y, Quackenbush EJ, Berman MA, Collins-Racie LA, Mi S, Reilly C, Lo D, Jacobs KA, Dorf ME: Identification of a new mouse β-chemokine, TC A-4, with activity on T lymphocytes and mesangial cells. J Immunol 1997;159:5671–5679.
Fan L, Reilly CR, Luo Y, Dorf ME, Lo D: Cutting Edge: Ectopic expression of the chemokine TCA 4/SLC is sufficient to trigger lymphoid neogenesis. J Immunol 2000;164:(April 15).
Lo D, Aftahi N, Reilly CR, Neal H, Sim B-C, Gascoigne NRJ, Kono D, Wu A, Schulman S, Scott B: Mapping genes regulating lymphocyte function: correlations with autoimmunity? in Theofilopoulos An (ed): Genes and Genetics of Autoimmunity (New Series: Current Directions in Autoimmunity) New York, Karger, 199, pp 226–246.
Sim B, Aftahi N, Reilly CR, Bogen B, Schwartz RH, Gascoigne NR, Lo D: Thymic skewing of the CD4/CD8 ratio maps with the T cell receptor α-chain locus. Curr Biol 1998;8:701–704.
Sim B-C, Lo D, Gascoigne NRJ: Preferential expression of TCR Vα, regions in CD4/CD8 subsets: class discrimination or co-receptor
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lo, D. Immune regulation. Immunol Res 21, 239–246 (2000). https://doi.org/10.1385/IR:21:2-3:239
Issue Date:
DOI: https://doi.org/10.1385/IR:21:2-3:239