Abstract
Background. Bile-duct and gallbladder carcinomas are rare cancers. Once they have spread beyond the point of surgical resectability, no therapies have shown meaningful long-term benefit. These cancers are typically refractory to standard chemotherapy agents. Based on preclinical work showing activity of CPT-11, we performed a phase II trial to assess its activity in patients with bile-duct or gallbladder carcinomas.
Methods. Patients with histologic or cytologic evidence of locally advanced or metastatic bile-duct or gallbladder carcinoma were potentially eligible for this study. Patients meeting study eligibility and who signed an informed consent were given CPT-11 125 mg/m2 weekly for 4 wk followed by a 2-wk break from therapy. The starting dose of CPT-11 was later reduced to 100 mg/m2 grade IV toxicity. Patients continued on treatment if they showed evidence of benefit and tolerated therapy.
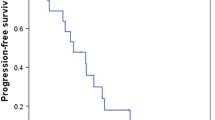
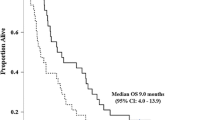
Results. A total of 39 patients were enrolled, and 36 were evaluable. The overall confirmed response rate was 8%. One CR and two PRs were seen. A high frequency of toxicity was seen. However, no unusual or unexpected toxicities occurred.
Conclusion. CPT-11 is ineffective therapy for patients with locally advanced or metastatic bile-duct or gallbladder carcinoma.
Similar content being viewed by others
References
Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin 2001;51:15–36.
Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 1995;75:171–190.
Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. histologic types, stage of disease, grade, and survival rates. Cancer 1992;70:1493–1497.
Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the extrahepatic bile ducts. Histologic types, stage of disease, grade, and survival rates. Cancer 1992;70:1498–1501.
Farley DR, Weaver AL, Nagorney DM. “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc 1995;70:425–429.
Takada T, Kato H, Matsushiro T, Nimura Y, Nagakawa T, Nakayama T. Comparison of 5-fluorouracil, doxorubicin and mitomycin C with 5-fluorouracil alone in the treatment of pancreatic-biliary carcinomas. Oncology 1994;51:396–400.
Taal BG, Audisio RA, Bleiberg H, Blijham GH, Neijt JP, Veenhof CH, et al. Phase II trial of mitomycin C (MMC) in advanced gallbladder and biliary tree carcinoma. An EORTC Gastrointestinal Tract Cancer Cooperative Group Study. Ann Oncol 1993;4:607–609.
Falkson G, MacIntyre JM, Moertel CG. Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer 1984;54:965–969.
Okada S, Ishii H, Nose H, Yoshimori M, Okusaka T, Aoki K, et al. A phase II study of cisplatin in patients with biliary tract carcinoma. Oncology 1994;51:515–517.
Jones DV, Jr., Lozano R, Hoque A, Markowitz A, Patt YZ. Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol 1996;14:2306–2310.
Kajanti M, Pyrhonen S. Epirubicin-sequential methotrexate-5-fluorouracil-leucovorin treatment in advanced cancer of the extrahepatic biliary system. A phase II study. Am J Clin Oncol 1994;17:223–226.
Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10.
Duffy DE, Santner TJ. Confidence intervals for a binomial parameter based on multistage tests. Biometrics 1987;43:81–93.
Kaplan E, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958;53:457–481.
Cox DR. Regression models and life tables. J R Stat Soc (B) 1972;74:187–220.
SAS/STAT user’s guide. Version 7, Cary (NC). SAS Institute, Inc. 1989.
De Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med 1999;341:1368–1378.
Sanz-Altamira PM, O’Reilly E, Stuart KE, Raeburn L, Steger C, Kemeny NE, et al. A phase II trial of irinotecan (CPT-11) for unresectable biliary tree carcinoma. Ann Oncol 2001;12:501–504.
Author information
Authors and Affiliations
Corresponding author
Additional information
Additional participating institutions include: CentraCare Clinic, St. Cloud, MN 56301 (Harold E. Windschitl, MD); Saskatchewan Cancer Centre, Saskatoon, Saskatchewan, Canada S7N 4H4, Allan Blair Cancer Centre, Regina, Saskatchewan, Canada S4T 7T1 (Muhammad Salim, MD); Toledo Community Hospital Oncology Program CCOP, Toledo, OH (Paul L. Schaefer, MD); Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882 (Shaker R. Dakhil, MD); Scottsdale CCOP, Scottsdale, AZ 85259-5404 (Tom R. Fitch, MD); Ann Arbor Regional CCOP, Ann Arbor, MI 48106 (Philip J. Stella, MD); Medcenter One Health Systems, Bismarck, ND 58506 (Ferdinand Addo, MD); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, MD); Meritcare Hospital CCOP, Fargo, ND 58122 (Ralph Levitt, MD); Altru Health Systems, Grand Forks, ND 58201 (Daniel J. Walsh, MD); Siouxland Hematology-Oncology Associates, Sioux City, IA 51105 (John C. Michalak, MD); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, MD); Missouri Valley Cancer Consortium, Omaha, NE 68131 (James A. Mailliard, MD).
Rights and permissions
About this article
Cite this article
Alberts, S.R., Fishkin, P.A., Burgart, L.J. et al. CPT-11 for bile-duct and gallbladder carcinoma. Int J Gastrointest Canc 32, 107–114 (2002). https://doi.org/10.1385/IJGC:32:2-3:107
Issue Date:
DOI: https://doi.org/10.1385/IJGC:32:2-3:107