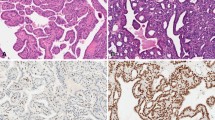
Abstract
The morphologic distinction of benign and malignant thyroid follicular lesions can sometimes be challenging, therefore an immunohistochemical marker to aid in this distinction would be useful. β-Catenin is one such potential marker. It is part of a membrane-bound cell growth-signaling complex that plays a role in cell adhesion, as well as in promotion of growth through activation of the Wnt signaling pathway. Oncogenic signaling occurs when β-catenin is released, accumulates in the cytoplasm, translocates into the nucleus, and promotes transcription of genes including bcl-1 (cyclin D1) and c-myc that induce cell proliferation.
Paraffin blocks from 133 thyroidectomy specimens were stained with monoclonal antibodies reactive with β-catenin and cyclin D1. These included 53 cases of papillary thyroid carcinoma (PTC), 46 cases of follicular variant of papillary carcinoma (FVPC), 10 cases of follicular carcinoma (FC), and 24 cases of follicular adenoma (FA). Tissue from six normal thyroid specimens served as a control. The malignant lesions (PTC, FC, and FVPC) expressed strong cytoplasmic/nuclear staining and minimal residual membranous staining in 87%, 80%, and 71% of cases, respectively. In contrast, all normal thyroid tissue and 79% of FAs showed strong membranous reactivity with very minimal cytoplasmic staining. Interestingly, in 83% of PTC cases and 20% FVPCs, the intranuclear inclusions were distinctly β-catenin positive. Cyclin D1 over expression correlated with cytoplasmic relocalization of β-catenin in almost all cases, and no evidence of cyclin D1 gene amplification was observed.
β-Catenin can be of a diagnostic utility for thyroid lesions, because it highlights intranuclear inclusions in PTC, and shifts from a membranous localization to a cytoplasmic localization in malignant lesions. We speculate that the localization of β-catenin in intranuclear inclusions may reflect a cytoskeletal remodeling activity of β-catenin that is functionally significant for the PTC pathway.
Similar content being viewed by others
References
Khan A, Nose V. Pathology of the thyroid gland. In Lloyd RV, ed. Endocrine pathology: differential diagnosis and molecular advances, 1st ed. Totawa, NJ: Humana Press 2004.
LiVolsi V, Montone K, Sack M. Thyroid Disease. In Sternberg S, ed. Diagnostic surgical pathology, 3rd ed. Philadelphia, Lippincott Williams & Wilkins, 1999.
Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun 2(2):77–85, 1999.
Rocha AS, Soares P, Seruca R, et al. Abnormalities of the E-cadherin/catenin adhesion complex in classical papillary thyroid carcinoma and in its diffuse sclerosing variant. J Pathol 194(3):358–366, 2001.
Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev 18(1):15–30, 1999.
Helmbrecht K, Kispert A, von Wasielewski R, et al. Identification of a Wnt/beta-catenin signaling pathway in human thyroid cells. Endocrinology 142(12):5261–5266, 2001.
Miyake N, Maeta H, Horie S, et al. Absence of mutations in the beta-catenin and adenomatous polyposis coli genes in papillary and follicular thyroid carcinomas. Pathol Int 51(9):680–685, 2001.
Israsena N, Hu M, Fu W, et al. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Developmental Biology 268:220–231, 2004.
Waterman-Storer CM, Salmon WC, Salmon ED. Feedback interactions between cell-cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells. Mol Biol Cell 11(7):2471–2483, 2000.
Fischer AH, Taysavang P, Weber C, et al. Nuclear envelope organization in papillary thyroid carcinoma. Histol Histopath 16:1–14, 2001.
Fischer AH, Taysavang P, Jhiang SM. Nuclear envelope irregularity is induced by RET/PTC during interphase. Am J Pathol 163:1091–1100, 2003.
Kapran Y, Ozbey N, Molvalilar S, et al. Immunohistochemical detection of E-cadherin, alpha- and beta-catenins in papillary thyroid carcinoma. J Endocrinol Invest 25(7):578–585, 2002.
Bohm J, Niskanen L, Kiraly K, et al. Expression and prognostic value of alpha-, beta- and gamma-catenins in differentiated thyroid carcinoma. J Clin Endocrinol Metab 85(12):4806–4811, 2000.
Oyama T. A histopathological, immunohistochemical and ultrastructural study of intranuclear cytoplasmic inclusions in thyroid papillary carcinoma. Virchows Arch A Pathol Anat Histopathol 414(2):91–104, 1989.
Saiz AD, Olvera M, Rezk S, et al. Immunohistochemical expression of cyclin D1, E2F-1, and Ki-67 in benign and malignant thyroid lesions. J Pathol 198(2):157–162, 2002.
Van Aken E, De Wever O, Correia da Rocha AS, et al. Defective E-cadherin/catenin complexes in human cancer. Virchows Arch 439(6):725–751, 2001.
Huiping C, Kristjansdottir S, Jonasson JG, et al. Alterations of E-cadherin and beta-catenin in gastric cancer. BMC Cancer 1(1):16, 2001. [Epub Oct 29, 2001].
Meirmanov S, Nakashima M, Kondo H, et al. Correlation of cytoplasmic beta-catenin and cyclin D1 overexpression during thyroid carcinogenesis around Semipalatinsk nuclear test site. Thyroid 13(6):537–545, 2003.
Kawanishi J, Kato J, Sasaki K, et al. Dysfunction of E-cadherin due to mutation of beta-catenin in a scirrhous gastric cancer cell line. Nippon Rinsho 53(7):1590–1594, 1995.
Miyoshi Y, Iwao K, Nagasawa Y, et al. Activation of the beta-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res 58(12):2524–2527, 1998.
Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res 58(12):2520–2523, 1998.
Rubinfeld B, Robbins P, El Gamil M, et al. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 275(5307):1790–1792, 1997.
Lin SY, Xia W, Wang JC, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 97(8):4262–4266, 2000.
Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-T cf signaling in colon cancer by mutations in beta-catenin or APC. Science 275(5307):1787–1790, 1997.
Ishigaki K, Namba H, Nakashima M, et al. Aberrant localization of beta-catenin correlates with overexpression of its target gene in human papillary thyroid cancer. J Clin Endocrinol Metab 87(7):3433–3440, 2002.
Garcia-Rostan G, Camp RL, Herrero A, et al. Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol 158(3):987–996, 2001.
Garcia-Rostan G, Tallini G, Herrero A, et al. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res 59(8):1811–1815, 1999.
Nakashima M, Meirmanov S, Naruke Y, et al. Cyclin D1 overexpression in thyroid tumors from a radio-contaminated area and its correlation with Pin1 and aberrant beta-catenin expression. J Pathol 202(4):446–455, 2004.
Natsume H, Sasaki S, Kitagawa M, et al. Beta-catenin/T cf-1-mediated transactivation of cyclin D1 promoter is negatively regulated by thyroid hormone. Biochem Biophys Res Commun 309(2):408–413, 2003.
Soderstrom N, Biorklund A. Intranuclear cytoplasmic inclusions in some types of thyroid cancer. Acta Cytol 17(3):191–197, 1973.
Chhieng DC, Ross JS, McKenna BJ. CD44 immunostaining of thyroid fine-needle aspirates differentiates thyroid papillary carcinoma from other lesions with nuclear grooves and inclusions. Cancer 81(3):157–162, 1997.
Lew W, Orell S, Henderson DW. Intranuclear vacuoles in nonpapillary carcinoma of thyroid: a report of three cases. Acta Cytol 28:581–586, 1984.
Kini SR, Guide to clinical aspiration biopsy: thyroid. 1st ed. New York: Igaku-Shoin, 1987.
Fiorella RM, Isley W, Miller LK, et al. Multinodular goiter of the thyroid mimicking malignancy: diagnostic pitfalls in fine needle aspiration. Diagn Cytopathol 9:351–357, 1993.
Gamachi A, Kashima K, Daa T, et al. Aberrant intranuclear localization of biotin, biotin-binding enzymes, and beta-catenin in pregnancy-related endometrial and morule-associated neoplastic lesions. Mod Pathol 16(11):1124–1131, 2003.
Ligon LA, Karki S, Tokito M, et al. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol 3(10):913–917, 2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezk, S., Brynes, R.K., Nelson, V. et al. β-Catenin expression in thyroid follicular lesions: Potential role in nuclear envelope changes in papillary carcinomas. Endocr Pathol 15, 329–337 (2004). https://doi.org/10.1385/EP:15:4:329
Issue Date:
DOI: https://doi.org/10.1385/EP:15:4:329