Abstract
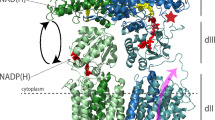
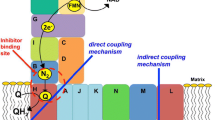
During aerobic growth of Escherichia coli, nicotinamide adenine dinucleotide (NADH) can initiate electron transport at either of two sites: Complex I (NDH-1 or NADH: ubiquinone oxidoreductase) or a single-subunit NADH dehydrogenase (NDH-2). We report evidence for the specific coupling of malate dehydrogenase to Complex I. Membrane vesicles prepared from wild type cultures retain malate dehydrogenase and are capable of proton translocation driven by the addition of malate+NAD. This activity was inhibited by capsaicin, an inhibitor specific to Complex I, and it proceeded with deamino-NAD, a substrate utilized by Complex I, but not by NDH-2. The concentration of free NADH produced by membrane vesicles supplemented with malate+NAD was estimated to be 1 μM, while the rate of proton translocation due to Complex I was consistent with a some what higher concentration, suggesting a direct transfer mechanism. This interpretation was supported by competition assays in which inactive mutant forms of malate dehydrogenase were able to inhibit Complex I activity.
These two lines of evidence indicate that the direct transfer of NADH from malate dehydrogenase to Complex I can occur in the E. coli system.
Similar content being viewed by others
References
Brandt, U. (1998) Special issue-structure and function of complex I-preface. Biochim. Biophys. Acta 1364, 85–86.
Matsushita, K., Ohnishi, T., and Kaback, H. R. (1987) NADH-ubiquinone oxidoreductases of the Escherichia coli aerobic respiratory chain. Biochemistry 26, 7732–7737.
Calhoun, M. W., Oden, K. L., Gennis, R. B., de Mattos, M. J., and Neijssel, O. M. (1993) Energetic efficiency of Escherichia coli: effects of mutations in components of the aerobic respiratory chain. J. Bacteriol. 175, 3020–3025.
Steuber, J. (2001) Na(+) translocation by bacterial NADH: quinone oxidoreductases: an extension to the complex-I family of primary redox pumps. Biochim. Biophys. Acta 1505, 45–56.
Barnes, S. J. and Weitzman, P. D. (1986) Organization of citric acid cycle enzymes into a multienzyme cluster. FEBS Lett. 201, 267–270.
Robinson, J. B., Jr., Inman, L., Sumegi, B., and Srere, P. A. (1987) Further characterization of the Krebs tricarboxylic acid cycle metabolon. J. Biol. Chem. 262, 1786–1790.
Velot, C., Mixon, M. B., Teige, M., and Srere, P. A. (1997) Model of a quinary structure between Krebs TCA cycle enzymes: a model for the metabolon. Biochemistry 36, 14271–14276.
Sumegi, B. and Srere, P. A. (1984) Complex I binds several mitochondrial NAD-coupled dehydrogenases. J. Biol. Chem. 259, 15040–15045.
Srivastava, D. K. and Bernhard, S. A. (1986) Metabolite transfer via enzyme-enzyme complexes. Science 234, 1081–1086.
Cori, C. F., Velick, S. F., and Cori, G. T. (1950) The combination of diphosphopyridine nucleotide with glyceraldehyde phosphate dehydrogenase. Biochim. Biophys. Acta 4, 160–169.
Mahler, H. R. and Elowe, D. (1954) Interaction between glyceraldehyde phosphate dehydrogenase and DPNH-cytochrome reductase. Biochim. Biophys. Acta 14, 100–107.
Nygaard, A. P. and Rutter, W. J. (1956) Interaction of pyridine-nucleotide linked enzymes. Acta Chem. Scand. 10, 37–48.
Davies, D. D., Teixeira, A., and Kenworthy, P. (1972) The stereospecificity of nicotinamide-adenine dinucleotide-dependent oxidoreductases from plants. Biochem. J. 127, 335–343.
Ovadi, J., Huang, Y., and Spivey, H. O. (1994) Binding of malate dehydrogenase and NADH channelling to complex I. J. Mol. Recognit. 7, 265–272.
Fukushima, T., Decker, R. V., Anderson, W. M., and Spivey, H. O. (1989) Substrate channeling of NADH and binding of dehydrogenases to complex I. J. Biol. Chem. 264, 16483–16488.
Ernster, L., Hoberman, H. D., Howard, R. L., King, T. E., Lee, C. P., Mackler, B., et al. (1965) Stereospecificity of certain soluble and particulate preparations of mitochondrial reduced nicotinamide-adenine dinucleotide dehydrogenase from beef heart. Nature 207, 940–941.
Kotlyar, A. B., Maklashina, E., and Cecchini, G. (2004) Absence of NADH channeling in coupled reaction of mitochondrial malate dehydrogenase and complex I in alamethicin-permeabilized rat liver mitochondria. Biochem. Biophys. Res. Commun. 318, 987–991.
Srere, P. A. (1987) Complexes of sequential metabolic enzymes. Annu. Rev. Biochem. 56, 89–124.
Carroll, J., Fearnley, I. M., Shannon, R. J., Hirst, J., and Walker, J. E. (2003) Analysis of the subunit composition of complex I from bovine heart mitochondria. Mol. Cell Proteomics 2, 117–126.
Weidner, U., Geier, S., Ptock, A., Friedrich, T., Leif, H., and Weiss, H. (1993) The gene locus of the proton-translocating NADH: ubiquinone oxidoreductase in Escherichia coli. Organization of the 14 genes and relationship between the derived proteins and subunits of mitochondrial complex I. J. Mol. Biol. 233, 109–122.
Satoh, T., Miyoshi, H., Sakamoto, K., and Iwamura, H. (1996) Comparison of the inhibitory action of synthetic capsaicin analogues with various NADH-ubiquinone oxidoreductases. Biochim. Biophys. Acta 1273, 21–30.
Hall, M. D., Levitt, D. G., McAlister-Henn, L., and Banaszak, L. J. (1991) Purification and crystallization of recombinant Escherichia coli malate dehydrogenase. J. Mol. Biol. 220, 551–553.
Amarneh, B. and Vik, S. B. (2003) Mutagenesis of subunit N of the Escherichia coli Complex I. Identification of the initiation codon and the sensitivity of mutants to decylubiquinone. Biochemistry 42, 4800–4808.
Kunkel, T. A. (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. USA 82, 488–492.
Voet, D. and Voet, J. G. (1995) Biochemistry, 2nd Ed., John Wiley & Sons, Inc., New York.
Bogachev, A. V., Murtazina, R. A., and Skulachev, V. P. (1996) H+/e− stoichiometry for NADH dehydrogenase I and dimethyl sulfoxide reductase in anaerobically grown Escherichia coli cells. J. Bacteriol. 178, 6233–6237.
Stolpe, S. and Friedrich, T. (2004) The Escherichia coli NADH: ubiquinone oxidoreductase (complex I) is a primary proton-pump but may be capable of secondary sodium antiport. J. Biol. Chem. 279, 18377–18383.
Steuber, J., Schmid, C., Rufibach, M., and Dimroth, P. (2000) Na+ translocation by complex I (NADH: quinone oxidoreductase) of Escherichia coli. Mol. Microbiol. 35, 428–434.
Yagi, T. (1990) Inhibition by capsaicin of NADH-quinone oxidoreductases is correlated with the presence of energy-coupling site 1 in various organisms. Arch. Biochem. Biophys. 281, 305–311.
Park, S. J., Cotter, P. A., and Gunsalus, R. P. (1995) Regulation of malate dehydrogenase (mdh) gene expression in Escherichia coli in response to oxygen, carbon, and heme availability. J. Bacteriol. 177, 6652–6656.
Calhoun, M. W. and Gennis, R. B. (1993) Demonstration of separate genetic loci encoding distinct membrane-bound respiratory NADH dehydrogenases in Escherichia coli. J. Bacteriol. 175, 3013–3019.
Geck, M. K. and Kirsch, J. F. (1999) A novel, definitive test for substrate channeling illustrated with the aspartate aminotransferase/malate dehydrogenase system. Biochemistry 38, 8032–8037.
Boernke, W. E., Millard, C. S., Stevens, P. W., Kakar, S. N., Stevens, F. J., and Donnelly, M. I. (1995) Stringency of substrate specificity of Escherichia coli malate dehydrogenase. Arch. Biochem. Biophys. 322, 43–52.
Hall, M. D., and Banaszak, L. J. (1993) Crystal structure of a ternary complex of Escherichia coli malate dehydrogenase citrate and NAD at 1.9 A resolution. J. Mol. Biol. 232, 213–222.
Leif, H., Sled, V. D., Ohnishi, T., Weiss, H., and Friedrich, T. (1995) Isolation and characterization of the proton-translocating NADH: ubiquinone oxidoreductase from Escherichia coli. Eur. J. Biochem. 230, 538–548.
Guénebaut, V., Schlitt, A., Weiss, H., Leonard, K., and Friedrich, T. (1998) Consistent structure between bacterial and mitochondrial NADH: ubiquinone oxidoreductase (complex I). J. Mol. Biol. 276, 105–112.
Wright, S. K., Zhao, F. J., Rardin, J., Milbrandt, J., Helton, M., and Furumo, N. C. (1995) Mechanistic studies on malte dehydrogenase from Escherichia coli. Arch. Biochem. Biophys. 321, 289–296.
Silverstein, E. and Sulebele, G. (1969) Catalytic mechanism of pig heart mitochondrial malate dehydrogenase studied by kinetics at equilibrium. Biochemistry 8, 2543–2550.
van der Rest, M. E., Frank, C., and Molenaard, D. (2000) Functions of the membrane-associated and cytoplasmic malate dehydrogenases in the citric acid cycle of Escherichia coli. J. Bacteriol. 182, 6892–6899.
Srivastava, D. K. and Bernhard, S. A. (1987) Mechanism of transfer of reduced nicotinamide adenine dinucleotide among dehydrogenases. Transfer rates and equilibria with enzyme-enzyme complexes. Biochemistry 26, 1240–1246.
Srivastava, D. K. and Bernhard, S. A. (1984) Direct transfer of reduced nicotinamide adenine dinucleotide from glyceraldehyde-3-phosphate dehydrogenase to liver alcohol dehydrogenase. Biochemistry 23, 4538–4545.
Srivastava, D. K., Smolen, P., Betts, G. F., Fukushima, T., Spivey, H. O., and Bernhard, S. A. (1989) Direct transfer of NADH between α-glycerol phosphate dehydrogenase and lactate dehydrogenase: factor misinterpretation?. Proc. Natl. Acad. Sci. USA, 86, 6464–6468.
Spehr, V., Schlitt, A., Scheide, D., Guénebaut, V., and Friedrich, T. (1999) Overexpression of the Escherichia coli nuo-operon and isolation of the overproduced NADH:ubiquinone oxidoreductase (complex I). Biochemistry 38, 16261–16267.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amarneh, B., Vik, S.B. Direct transfer of NADH from malate dehydrogenase to Complex I in Escherichia coli . Cell Biochem Biophys 42, 251–261 (2005). https://doi.org/10.1385/CBB:42:3:251
Issue Date:
DOI: https://doi.org/10.1385/CBB:42:3:251