Abstract
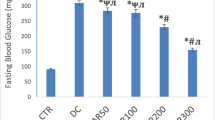
It is known that selenium compounds can restore some metabolic parameters in experimental diabetes. However, as there are no, clear data about their effects on the altered antioxidant defense system of the diabetic heart, we aimed to investigate whether these beneficial effects extend to the alterations of some enzyme activities, which play important roles in antioxidant defense system. Diabetes was induced by streptozotocin (50 mg/kg body weight) and rats were then treated with sodium selenite (5 μmol/kg/d) for 4 wk. Sodium selenite treatment of the diabetic rats significantly restored the altered activities of glutathione- S-transferase, glucose-6-phosphate dehydrogenase, and 6-phosphogluconate dehydrogenase, which are involved in the glutathione metabolism of the heart, but slightly but significantly decreased the high blood glucose level. In summary, the present study suggests that the beneficial effects of sodium selenite treatment appears to be the result of the restoration altered activities of the antioxidant enzymes in diabetic heart tissue.
Similar content being viewed by others
References
F. S. Fein, L. B. Kornstein, J. E. Strobeck, J. M. Capasso, and E. H. Sonnenblick, Altered myocardial mechanics in diabetic rats, Circ. Res. 47, 922–933 (1980).
T. J. Regan, M. M. Lyons, S. S. Ahmed, et al., Evidence for cardiomyopathy in familial diabetes mellitus, J. Clin. Invest. 60, 885–899 (1977).
Y. Shechter, Insulin-mimetic effects on vanadate. Possible implications for future treatment of diabetes, Diabetes 1, 1–5 (1990).
J. H. McNeill, H. L. M. Delgatty, and M. L. Battell, Insulinlike effects of sodium selenate in streptozotocin-induced diabetic rats. Diabetes 40, 1675–1678 (1991).
R. Ghosh, B. Mukherjee, and M. Chatterjee, A novel effect of selenium on streptozotocin-induced diabetic mice, Diabetes Res. 25, 165–171 (1994).
E. A. Berg, J. Y. Wu, L. Campbell, M. Kagey, and S. R. Stapleton, Insulin-like effects of vanadate and selenate on the expression of glucose-6-phosphate dehydrogenase and fatty acid synthase in diabetic rats, Biochimie 77, 919–924 (1995).
D. J. Becker, B. Reul, A. T. Ozcelikay, J. P. Buchet, J. C. Henquin, and S. M. Brichard, Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycogenic and gluconeogenic enzymes in diabetic rats, Diabetologia 39, 3–11 (1996).
S. R. Stapleton, G. Garlock, L. Foellmi-Adam, and R. F. Kletzien, Selenium: potent stimulator of tyrosyl phosphorylation and activator of MAP kinase, Biochem. Biophys. Acta 1355, 259–269 (1997).
B. Mukherjee, S. Anbszhagan, A. Roy, R. Ghosh, and M. Chatterjee, Novel implications of the potential role of selenium on antioxidant status in streptozotocin-induced diabetic mice, Biomed. Pharmacother. 52, 89–95 (1998).
M. L. Battell, H. L. M. Delgatty, and J. H. McNeill, Sodium selenate corrects glucose tolerance and heart function in STZ diabetic rats, Mol. Cell. Biochem. 179, 27–34 (1998).
M. Ayaz, B. Can, S. Ozdemir, and B. Turan, Protective effect of selenium treatment on diabetes-induced myocardial structural alterations, Biol. Trace Element Res. 89, 215–226 (2002).
G. Ersoz, A. Yakaryilmaz, and B. Turan, Effect of sodium selenite treatment on platelet aggregation of streptozotocin-induced diabetic rats, Thromb. Res. 11, 363–367 (2003).
M. Ayaz, S. Ozdemir, M. Ugur, G. Vassort, and B. Turan, Effects of selenium on altered mecnanicai and electrical cardiac activities of diabetic rat, Arch. Biochem. Biophys. 426, 83–90 (2004).
O. Ezaki, The insulin-like effects of selenate in rat adipocytes, J. Biol. Chem. 265, 1124–1130 (1990).
E. Heart and C. K. Sung, Insulin-like and non-insulin-like selenium actions in 3T3-L1 adipocytes, J. Cell. Biochem. 88, 719–731 (2003).
J. T. Rotruck, A. L. Pope, H. E. Ganther, A. B. Swanson, D. G. Hafemen, and W. G. Hoekstra, Selenium: biochemical role as a component of glutathione peroxidase, Science 179, 588–590 (1973).
L. D. Koller and J. H. Exon, The two faces of selenium-deficiency and-toxicity are similar in animals and man, Can. J. Vet. Res. 50, 297–306 (1986).
J. Neve, Physiological and nutritional importance of selenium, Experienta 47, 187–193 (1991).
D. H. Mak, S. P. Ip, P. C. Li, M. K. Poon, and K. M. Ko, Alterations in tissue glutathione system in streptozotocin-induced diabetic rats, Mol. Cell. Biochem. 162(2), 153–158 (1996).
K. Doi, F. Sawada, G. Toda, et al., Alteration of antioxidants during the progression of heart disease in streptozotocin-induced diabetic rats. Free Radical Res. 34(3), 251–261 (2001).
K. Betke, H. N. Brewer, L. Kirkman, et al., Standardized method for G-6-PD assay of haemolysates, WHO Tech. Rep. Ser. 366, 30–32 (1967).
B. M. F. Pearse and M. A. Rosemeyer, 6-Phosphogluconate dehydrogenase from human erythrocytes, in Methods in Enzymology, Volume XLI, Academic S. R. Colowich and N. O. Kaplan, eds., London, p. 220 (1975).
W. H. Habig and W. B. Jakoby, Glutathione transferase (human placenta), Methods Enzymol. 77, 218–231 (1981).
M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding, Anal. Biochem. 72, 248–254 (1976).
G. J. Rozanski, Z. Xu, S. P. Didion, and W. G. Mayhan, Metabolic basis of decreased transient outwark K+ current in ventricular myocytes from rats with experimental heart failure, Circulation 96, 1–7 (1997).
G. R. Lee, T. C. Bithell, J. Foerster, J. W. Athens, and J. N. Lukens, Glucose-6-phosphate dehydrogenase deficiency and related deficiencies involving the pentose phosphate pathway and glutathione metabolism, in Wintrobe's Clinical Hematology, G. R. Lee, ed., Lea and Febiger, Philadelphia, pp. 1006–1016 (1993).
T. P. Mulder, D. A. Court, and W. H. Peters, Variability of glutathione-S-transferase in human liver and plasma, Clin. Chem. 45, 355–359 (1999).
Z. Xu, K. P. Patel, M. F. Lou, and G. J. Rozanski, Up-regulation of K+ channels in diabetic rat ventricular myocytes by insulin and glutathione, Cardiovasc. Res. 53, 80–88 (2002).
C. Douillet, M. Bost, M. Accominotti, F. Borson-Chazot, and M. Ciavatti, Effect of selenium and vitamin E supplements on tissue lipids, peroxides, and fatty acid distribution in experimental diabetes, Lipids 33(4), 393–399 (1998).
W. E. Connor, Importance of n-3 fatty acids in health and disease, Am. J. Clin. Nutr. 71, 171S-175S (2000).
T. Hunkar, F. Aktan, A. Ceylan, and C. Karasu, Effects of cod liver oil on tissue antioxidant pathways in normal and streptozotozin-diabetic rats, Cell. Biochem. Funct. 20, 297–302 (2002).
C. Furnsinn, R. Englisch, K. Ebner, P. Nowotny, C. Volge, and W. Waldhausl, Insulin-like vs non-insulin like stimulation of glucose metabolism by vanadium, tungsten and selenium compounds in rat muscle, Life Sci. 59, 1989–2000 (1996).
C. Gocmen, A. Secilmis, E. K. Kumcu, et al., Effects of vitamin E and sodium selenate on neurogenic and endothelial relaxation of corpus cavernosum in the diabetic mouse, Eur. J. Pharmacol. 398, 93–98 (2000).
L. H. Foster and S. Sumar, Selenium in health and disease: a review, Crit. Rev. Food Sci. Nutr. 37, 211–228 (1997).
L. Rao, B. Puschner, and T. A. Prolla, Gene expression profiling of low selenium status in the mouse intestine: transcriptional activation of genes linked to DNA damage, cell cycle control and oxidative stress, J. Nutr. 131(12), 3175–3181 (2001).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ulusu, N.N., Turan, B. Beneficial effects of selenium on some enzymes of diabetic rat heart. Biol Trace Elem Res 103, 207–215 (2005). https://doi.org/10.1385/BTER:103:3:207
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:103:3:207