Abstract
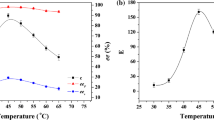
A lipase-catalyzed enantioselective transesterification process was developed for the synthesis of (S)-naproxen 2-N-morpholinoethyl ester prodrug from racemic 2,2,2-trifluoroethyl naproxen ester in organic solvents. By selecting isooctane and 37°C as the best solvent and temperature, the apparent fits of the initial conversion rates for transesterification and hydrolysis side reaction suggest a ping-pong Bi-Bi enzymatic mechanism with the alcohol as a competitive enzyme inhibitor. Improvements in the initial conversion rate and the productivity for the desired (S)-ester product were obtained after comparing with the result of an enantioselective esterification process. Studies of water content in isooctane and alcohol containing various N,N-dialkylamino groups on the enzyme activity and enantioselectivity, as well as the recovery of (S)-ester product by using extraction, were also reported.
Similar content being viewed by others
References
Federsel, H. J. (1993), CHEMTECH 12, 24–33.
Margolin, A. J. (1993), Enzyme Microb. Technol. 15, 266–280.
Hutt, A. J. and Caldwell, J. (1984), Clin. Pharmacokinet. 9, 371–373.
Shanbhag, V. R., Crider, A. M., Gokhale, R., Harpalani, A., and Dick, R. M. (1992), J. Pharm. Sci. 81, 149–154.
Bundgaard, H. (1985), Design of Prodrugs, Elsevier, Amsterdam.
Nielsen, N. N. and Bundgaard, H. (1988), J. Pharm. Sci. 77, 285–298.
Tammara, V. K., Narurkar, M. M., Crider, A. M., and Khan, A. M. (1993), Pharm. Res. 10, 1191–1199.
Chang, C. S. and Tsai, S. W. (1997), Enzyme Microb. Technol. 20, 635–639.
Tsai, S. W., Lin, J. J., Chang C. S., and Chen, J. P. (1997), Biotechnol. Prog. 13, 82–88.
Chang, C. S. and Tsai, S. W. (1997), Appl. Biochem. Biotechnol., Part A: Enzyme Eng. Biotechnol. 68, 135–142.
Tsai, S. W. and Wei, H. J. (1993), J. Liq. Chromatogr. 16, 2993–3001.
Allenmark, S. and Ohlsson, A. (1992), Chirality 4, 98–102.
Nielsen, N. M. and Bundgaard, H. (1987), Int. J. Pharm. 39, 75–85.
Rizzi, M. P., Stylos, P., and Reuss, M. (1992), Enzyme Microb. Technol. 14, 709–714.
Chen, C. S., Fujimoto, Y., Girdaukas, G., and Sih, C. J. (1982), J. Am. Chem. Soc. 104, 7294–7299.
Tsai, S. W. and Wei, H. J. (1994), Biocatalysis 11, 33–45.
Dordick, J. S. (1990), in Applied Biocatalysis, vol. 1, Blanch, H. W. and Clark, D. S., eds., Marcel Dekker, New York, pp. 1–52.
Halling, P. (1994), Enzyme Microb. Technol. 16, 178–206.
Phillips, R. S. (1992), Enzyme Microb. Technol. 14, 417–419.
Palomer, A., Cabre, M., Ginesta, J., Mauleon, D., and Carganico, G. (1993), Chirality 5, 320–328.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, SW., Tsai, CS. & Chang, CS. Lipase-catalyzed synthesis of (S)-naproxen ester prodrug by transesterification in organic solvents. Appl Biochem Biotechnol 80, 205–219 (1999). https://doi.org/10.1385/ABAB:80:3:205
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:80:3:205