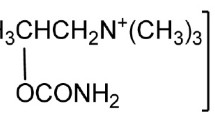Abstract
A liquid chromatographic method has been developed and validated for quantitative analysis of pipenzolate bromide (PP), its hydrolysis products, and phenobarbitone, sodium benzoate, and sodium saccharine. A 5-μm particle ODS column was used with acetonitrile–KH2PO4 (10 mm, pH 3.5) 40:60 (v/v), containing 5 mm heptanesulfonic acid sodium salt, as mobile phase. Quantitation was achieved by UV detection at 210 nm, on the basis of peak area. Forced degradation studies were performed on a bulk sample of PP using 0.1 M hydrochloric acid, 0.01 M sodium hydroxide, 0.33% hydrogen peroxide, heat (70 °C), and photolytic degradation. The proposed LC method was used to study the kinetics of acidic hydrolysis and pH-rate profiles of hydrolysis of PP in Britton–Robinson buffer solutions.






Similar content being viewed by others
References
Sweetman SC (ed) (2002) Martindale—the complete drug reference, 33rd edn. Pharmaceutical Press, London
Budavari S, Smith A, Kinneary JF (eds) (1996) The Merck index, 12th edn. Merck Research Laboratories
Stanley SMR, Foo HC (2006) J Chromatogr B Analyt Technol Biomed Life Sci 836:1–14. doi:10.1016/j.jchromb.2006.03.034
Yiu KCH, Ho ENM, Wan TSM (2004) Chromatographia 59:S45–S50. doi:10.1365/s10337-004-0233-9
Tang FPW, Leung GNW, Wan TSM (2001) Electrophoresis 22:2201–2209. doi:10.1002/1522-2683(20017)22:11<2201::AID-ELPS2201>3.0.CO;2-S
Ozkan SA, Erk N, Senturk Z (1999) Anal Lett 32:497–520. doi:10.1080/00032719908542836
Sabnis DS, Shirodkar MM (1985) Indian Drugs 23:50–51
International conference on harmonization (1993) Stability testing of new drug substances and products. ICH, Geneva
Snyder LR, Kirkland JJ, Glajch JL (1997) Practical HPLC method development, 2nd edn. Wiley, New York, p 317
European Agency for the Evaluation of Medical Products (1996) ICH Topic Q 2B Note for guidance on validation of analytical procedures: methodology CPMP/ICH/281/95
British Pharmacopoeia (2003) The Stationery Office, London
The United States Pharmacopeia 30 (2007) The National Formulary 25, United States Pharmacopeial Convention, INC. p 249–253
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadad, G.M. Validated, Stability-Indicating LC Method for Analysis of Pipenzolate Bromide and Its Hydrolysis Products. Chroma 68, 207–212 (2008). https://doi.org/10.1365/s10337-008-0715-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0715-2