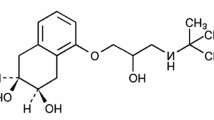
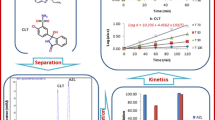
Abstract
A fast, sensitive and selective RP-HPLC method was developed for extensive investigation of nitroxinil’s stability. The stability of the studied drug was tested under different stress conditions, namely hydrolytic, oxidative, photolytic and thermal. Separation of nitroxinil and its degradation products was achieved in less than 5 min using Venusil XBP C18 (150 × 2.1 mm id, 5 um particle size) column and isocratic mobile phase composed of 0.1% triethylamine pH 2.5 (adjusted with phosphoric acid) and acetonitrile mixture in a ratio of (70:30; v/v). UV detection at 270 nm was employed for monitoring nitroxinil degradation behavior over a linearity range of 1–75 µg/mL. Plackett–Burman experimental design was adopted for robustness testing of the developed chromatographic method. LC-mass identification of nitroxinil’s hydrolytic and oxidative degradations was attempted, and the suggested mechanism was deduced. The proposed method was successfully applied in determination of the drug in raw material and pharmaceutical dosage form.







Similar content being viewed by others
References
A.C. Moffat, M.D. Osselton, B. Widdop, J. Watts, Clarke’s analysis of drugs and poisons, in Pharmaceuticals, Body Fluids and Postmortem Material, 4th. Pharmaceutical Press (2011)
British Pharmacopeia, Her Majesty’s Stationary Office, London, UK (2016)
W.J. Blanchflower, D.G. Kennedy, Determination of nitroxynil residues in tissues using high-performance liquid chromatography-thermospray mass spectrometry. Analyst 114, 1013–1015 (1989)
J.A. Tarbin, G. Shearer, High-performance liquid chromatographic method for the determination of the anthelmintic nitroxynil in cattle muscle tissue with on-line anion-exchange clean-up. J. Chromatogr. 613, 347–353 (1993)
M. Sakamoto, K. Takeba, T. Sasamoto et al., Determination of bithionol, bromophen, nitroxynil, oxyclozanide, and tribromsalan in milk with liquid chromatography coupled with tandem mass spectrometry. J AOAC Int 93, 1340–1346 (2010)
M. Whelan, Y. Bloemhoff, A. Furey et al., Investigation of the persistence of nitroxynil residues in milk from lactating dairy cows by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. J. Agric. Food Chem. 59, 7793–7797 (2011). https://doi.org/10.1021/jf202050r
K. Takeba, M. Matsumoto, Determination of nitroxynil in cow milk by reversed-phase high-performance liquid chromatography with dual- electrode coulometric detection. J. Chromatogr. 596, 67–71 (1992)
G. Shearer, Short Communication liquid chromatographic method for the determination of the anthelmintic nitroxynil in cattle muscle tissue with on-line anion-exchange. 3, 347–353 (1993)
M. Caldow, M. Sharman, M. Kelly et al., Multi-residue determination of phenolic and salicylanilide anthelmintics and related compounds in bovine kidney by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1216, 8200–8205 (2009). https://doi.org/10.1016/j.chroma.2009.04.008
K.M. Cooper, M. Whelan, M. Danaher et al., Stability during cooking of anthelmintic veterinary drug residues in beef. Food Addit. Contam. 28, 155 (2012). https://doi.org/10.1080/19440049.2010.542775
Sunil Kumar Yelamanchi, Useni Reddy Mallu, I.V. Kasi Viswanath, G.N. Murthy, RP-HPLC method development and validation for nitroxynil in active Pharmaceutical ingredient. J Pharm Sci researh 8, 967–973 (2016)
M.M. Ghoneim, M. El-ries, A.M. Hassanein, A.M. Abd-elaziz, Voltammetric assay of the anthelmintic veterinary drug nitroxynil in bulk form and formulation at a mercury electrode. J. Pharm. Biomed. Anal. 41, 1268–1273 (2006). https://doi.org/10.1016/j.jpba.2006.03.022
I.M. Traynor, C.S. Thompson, L. Armstrong et al., Determination of nitroxynil residues in tissues and bovine milk by immunobiosensor. Food Addit Contam Part A 30, 1115–1122 (2013). https://doi.org/10.1080/19440049.2013.781274
(2016) United State Pharmacopeia and National Formulary (USP39—NF34). Rockville,MD,
Speeding up your HPLC. http://www.chromacademy.com/chromatography-speeding-up-your-HPLC.html. Accessed 25 Mar 2017
Chemicalize. https://chemicalize.com/#/calculation. Accessed 25 Mar 2017
ReviewerGuidance, Validation of Chromatographic Methods, Center for Drug Evaluation and Research(CDER),FDA,. Rockville, MD (1994)
Y. Qiu, Y. Chen, L. Yu, R.V. Mantril, Developing Oral Dosage forms pharmaceutical theory and practice, 2nd edn. (Academic Press, Boca Raton, 2016)
F.A. Carey, R.J. Sundberg, Advanced Organic Chemistry, 5th edn. (Springer, Berlin, 2007)
J.E. Mcissac Jr., R.E. Bal Jr, E.J. Behrman, The mechanism of the base catalyzed conversion of nitrile to amides by hydrogen peroxide. J. Org. Chem. 3961, 3048–3050 (1971)
J. Ermer, J.H.M. Miller, Method validation in pharmaceutical analysis: A guide to best practice, first edit (WILEY-VCH Verlag GmbH & Co, KGaA, Weinheim, 2005)
Y. Vander Heyden, A. Nijhuis, J. Nijhuis et al., Guidance for robustness/ruggedness tests in method validation. J. Pharm. Biomed. Anal. 24, 723–753 (2001). https://doi.org/10.1016/S0731-7085(00)00529-X
Acknowledgment
The research was carried out using the materials, equipment and facilities of National Organization for Drug Control and Research, Ministry of Health, Egypt.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Soliman, M., Saad, A.S., Ismail, N.S. et al. A validated RP-HPLC method for determination of nitroxinil and investigation of its intrinsic stability. J IRAN CHEM SOC 18, 351–361 (2021). https://doi.org/10.1007/s13738-020-02030-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02030-w