Abstract
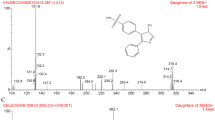
An analytical method based on liquid chromatography-tandem mass spectrometry (LC-MS-MS) was developed and validated for the determination of ezetimibe in human plasma. Ezetimibe and etoricoxib (internal standard) were extracted from the plasma by liquid-liquid extraction and separated on a C18 analytical column (50 × 3.0 mm I.D.) with acetonitrile:water (85:15, v/v) as mobile phase. Detection was carried out by positive electrospray ionization (ESI+) in multiple reaction monitoring (MRM) mode. The chromatographic separation was obtained within 2.0 min and was linear in the concentration range of 0.25–20ng mL−1 for free ezetimibe and of 1–300ng mL−1 for total ezetimibe. The mean extraction recoveries for free and total ezetimibe from plasma were 96.14 and 64.11%, respectively. Method validation investigated parameters such as linearity, precision, accuracy, specificity and stability, giving results within the acceptable range. The proposed method was successfully applied to the quantitation of ezetimibe and its glucuronide in human plasma to support clinical and pharmacokinetic studies. Moreover, the method was used for the quality control analysis of pharmaceutical dosage forms.
Similar content being viewed by others
References
Catapano AL (2001) Eur Heart J Supplements 3(Suppl E):E6–E10
Clader JW (2004) J Med Chem 47:1–9
Ezzet F, Krishna G, Wexler DB, Statkevich P, Kosoglou T, Batra VK (2001) Clin Ther 23:871–885
Davis HR (2004) International Congress Series 1262:243–246
Sistla R, Tata VSSK, Kashyap YV, Chandrasekar D, Diwan PV (2005) J Pharm Biomed Anal 39:517–522
Lee H (2005) J Liq Chrom Rel Tech 28:1161–1202
Trivedi RK, Kallem RR, Mullangi R, Srinivas NR (2005) J Pharm Biomed Anal 39:661–669
Guidance for Industry: Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug administration, Center for Drug Evaluation and Research, Rockville, MD, 2001
Author information
Authors and Affiliations
Corresponding author
Additional information
Revised: 4 January and 3 February 2006
Rights and permissions
About this article
Cite this article
Oliveira, P.R., Junior, L.B., Fronza, M. et al. Development and Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Ezetimibe in Human Plasma and Pharmaceutical Formulations. Chroma 63, 315–320 (2006). https://doi.org/10.1365/s10337-006-0749-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-006-0749-2