Abstract
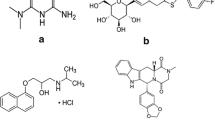
A sensitive and specific method based on liquid chromatographic–tandem mass spectrometric method (LC–MS/MS) has been developed and validated to determine plasma concentrations of olopatadine. Olopatadine and internal standard (IS, loratadine) from human plasma were extracted using solid-phase extraction. Chromatographic separation was achieved on a reversed-phase Capcellpak CR column using the isocratic mobile phase consisted of 70 % acetonitrile and 30 % water containing 10 mM ammonium acetate (adjusted to pH 4.0 with acetic acid). Acquisition was performed in multiple reaction monitoring mode by monitoring the transitions: m/z 337.92 → 164.80 for olopatadine and m/z 383.17 → 336.90 for IS. This method was fully validated. The calibration curve was linear over the concentration range from 0.2 to 100 ng/mL, and correlation coefficients (r 2) were greater than 0.99. The low limit of quantitation with a relative standard deviation below 20 % was 0.2 ng/mL. The intraday and interday precisions ranged 6.31–16.80 % and intraday and interday accuracies ranged 91.17–110.08 %. The devised method was successfully applied in a bioequivalence study of two formulations of olopatadine, Allotadine tablet and Allelock tablet in 26 healthy Korean volunteers following single oral administration.



Similar content being viewed by others
References
Abelson MB, Gomes PJ (2008) Olopatadine 0.2% ophthalmic solution: the first ophthalmic antiallergy agent with once-daily dosing. Expert Opin Drug Metab Toxicol 4(4):453–461
BA Calc 2002 software for windows (2002) KFDA. http://www.kfda.go.kr/
Chu NN, Chen WL, Xu HR, Li XN (2009) Pharmacokinetics of orally administered single—and multiple-dose olopatadine in healthy Chinese subjects: an open-label study. Clin Drug Investig 29(7):451–457
Fujimaki K, Lee XP, Kumazawa T, Sato J, Sato K (2006) Determination of some antiallergic drugs in human plasma by direct-injection high-performance liquid chromatography-tandem mass spectrometry. Forensic Toxicol 24:8–16
Fujita K, Magara H, Kobayashi H (1999) Determination of olopatadine, a new antiallergic agent, and its metabolites in human plasma by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J Chromatogr B 731:345–352
Korea Food & Drug Administration, Guidance for industry, Statistical Approaches to Establishing Bioequivalence, Bioequivalence Division, Pharmacology Department, National Institute of Toxicology Department (2005) http://www.kfda.go.kr/
Ohmori K, Hayashi K, Kaise T, Ohshima E, Kobayashi S, Yamazaki T, Mukouyama A (2002) Pharmacological, pharmacokinetic and clinical properties of olopatadine hydrochloride, new antiallergic drug. Jpn J Pharmacol 88(4):379–397
Ohmori K, Hasegawa K, Tamura T, Miyake K, Matsubara M, Masaki S, Karasawa A, Urayama N, Horikoshi K, Kajita J, Hasegawa M, Taniguchi K, Komada T, Kawamoto Y (2004) Properties of olopatadine hydrochloride, a new antiallergic/antihistamine drug. Arzneimittelforschung 54(12):809–829
Roland P, Ryan M, Wall G (2010) Olopatadine nasal spray for the treatment of seasonal allergic rhinitis in patients aged 6 years and older. Expert Opin Pharmacother 11(9):1559–1567
Rosenwasser LJ, O’Brien T, Weyne J (2005) Mast cell stabilization and anti-histamine effects of olopatadine ophthalmic solution: a review of pre-clinical and clinical research. Curr Med Res Opin 21(9):1377–1387
US Department of Health & Human Service, Food and Drug Administration, Guidance for Industry, Bioanalytical Method Validation (2001) US FDA. http://www.fda.gov/
World Medical Association (2000) Declaration of Helsinki: ethical principles for medical research involving human subjects. As amended by the 52nd World Medical Assembly, Edinburgh, Scotland
Zhu P, Wen YG, Fan XP, Zhou ZL, Fan RX, Chen JM, Huang KL, Zhu XL, Zhuang J (2011) A rapid and sensitive liquid chromatography-tandem mass spectrometry method for determination of olopatadine concentration in human plasma. J Anal Toxicol 35(2):113–118
Acknowledgments
All authors (S. W. Choi, J. S. Park, J. H. Ryu, M. J. Lee, S. V. Yim, K. T. Lee) declare that they have no conflict of interest. This work was supported by Ilhwa Co., Ltd., Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Soo-Wan Choi and Ju-Hee Ryu have equal contribution in this work.
Rights and permissions
About this article
Cite this article
Choi, SW., Ryu, JH., Park, JS. et al. Development and validation for the determination of olopatadine in human plasma by liquid chromatography–tandem mass spectrometry: application to a bioequivalence study of Ilhwa Allotadine tablet (olopatadine HCl 5 mg). Journal of Pharmaceutical Investigation 45, 285–292 (2015). https://doi.org/10.1007/s40005-015-0175-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-015-0175-2