Abstract
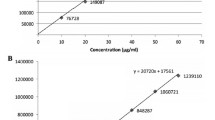
A new, simple, rapid and specific reversed-phase high-performance liquid chromatography (HPLC) method was developed and validated for the determination of fluvoxamine in pharmaceutical dosage forms. The HPLC separation was achieved on a C18 μ-Bondapack column (250 mm × 4.6 mm) using a mobile phase of acetonitrile–water (80:20, v/v) at a flow rate of 1 mL min−1. Proposed method is based on the derivatization of fluvoxamine with 1,2-naphthoquinone-4-sulphonic acid sodium salt (NQS) in borate buffer of pH 8.5 to yield a orange product. The HPLC method is based on measurement of the derivatized product using UV-visible absorbance detection at 450 nm. The method was validated for specificity, linearity, precision, accuracy, robustness. The degree of linearity of the calibration curves, the percent recoveries of fluvoxamine, the limit of detection and quantification, for the HPLC method were determined. The assay was linear over the concentration range of 45–145 ng mL−1 (r = 0.9999). Limit of detection and quantification for fluvoxamine were 15 and 50 ng mL−1, respectively. The results of the developed procedure (proposed method) for fluvoxamine content in tablets were compared with those by the official method. The method was found to be simple, specific, precise, accurate, reproducible and robust.



Similar content being viewed by others
References
The Merck Index (2001) 748:Fluvoxamine
Wilde MI, Plosker GL, Benfield P (1993) Drugs 46:896
Berzas Nevado JJ, Villaseñor Lierena MJ, Contento Salcedo AM, Aguas Nuevo E (2005) J Pharm Biomed Anal 38:52–594
Berzas JJ, Guiberteau C, Villaseñor MJ, Rodriguez V (2004) Anal Chim Acta 519:219–230
Skibinski R, Misztal G (2004) J Planar Chromatogr Mod TLC 17:224–228
Skibinski R, Misztal G (2001) Acta Poloniae Pharmaceutica Drug Res 58:97–100
Starczewska B, Mielech K (2000) J Pharm Biomed Anal 23:243–247
Berzas Nevado JJ, Contento Salcedo AM, Villaseñor Llerena MJ, Aguas Nuevo E (2000) Anal Chim Acta 417:169–176
Elmali F, Alpdogan G, Sungur S, Aycan S (2000) Turk J Chem 24:299–302
Misztal G, Skibinski R (1999) Acta Poloniae Pharm Drug Res 56:95–100
Foda NH (1995) J Liq Chromatogr 18:1591–1601
Atmaca S, Tatar S (1995) Acta Pharm Turc 37:33–3713
Atmaca S, Tatar S (1994) Pharmazie 49:458–459
Yasui-Furukori Inoue N, Kaneko YS, Otani K (2005) J Pharm Biomed Anal 37:121–125
Titier K, Castaing N, Scotto-Gomez E, Pehourcq F, Moore N, Molimard M (2003) Ther Drug Monit 25:581–587
Duverneuil C, de la Grandmaison GL, Mazancourt P, Alvarez JC (2003) Ther Drug Monit 25:565–573
Wong SH, Kranzler HR, Della Fera S, Fernandes R (1994) Biomed Chromatogr 8:278–282
Hartter S, Wetzel H, Hiemke C (1992) Clin Chem 38:2082–2086
Van Der Meersch-Mougeot V, Diquet B (1991) J Chromatogr 567:441–449
Foglia JP, Birder LA, Perel JM (1989) J Chromatogr B 495:295–302
Lhermitte M (1989) Biomed Chromatogr 3:177–179
Pullen RH, Fatmi AA (1992) J Chromatogr 574:101–107
Schweitzer C, Spahn H, Mutschler E (1986) J Chromatogr B 382:412–414
British Pharmacopoeia, Her Majesty’s Stationery Office, London (1998) 799–800
Validation of analytical procedures: Methodology ICH Harmonised Tripartite Guideline, Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on, 6 November (1996)
Shabir GA (2003) J Chromatogr A 987:57–66
British Pharmacopoeia (2001) 765
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulu, S.T. Determination and Validation of an LC Method for Fluvoxamine in Tablets. Chroma 64, 169–173 (2006). https://doi.org/10.1365/s10337-006-0016-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-006-0016-6