Abstract
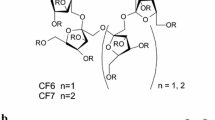
The enantiomeric separation of 12 chiral dihydrobenzofurans is reported on derivatized β-cyclodextrin stationary phases using high performance liquid chromatography. The hydroxypropyl β-cyclodextrin chiral stationary phase (CSP) with acetonitrile/water mobile phases was the most effective combination as it baseline separated 9 of the 12 compounds. The acetyl β-cyclodextrin and 2,3-dimethyl β-cyclodextrin CSPs were also effective in the reverse phase mode. The native β-cyclodextrin was far less effective than the non-aromatic derivatized CSPs. The aromatic functionalized CSPs showed no selectivity in the normal phase mode. Structural characteristics, such as substituent polarity and ring location, were important in the observed enantioselectivity.
Similar content being viewed by others
References
Cagniant P, Cagniant D (1975) Adv Heterocycl Chem 18:337–482
Mustafa A (1974) Benzofurans. In: Weissberger A, Taylor EC (eds) The chemistry of heterocyclic compounds. New York, pp. 452–462
Fancelli D, Caccia C, Fornaretto MG, McArthur R, Severino D, Vaghi F, Varai M (1996) Biomed Chem Lett 6:263–266
Chen C-H, Shaw C-Y, Chen C-C, Tsai Y-C, (2002) J Nat Prod 65:740–741
Yamaguchi S, Muro S, Kobayashi M, Miyazawa M, Hirai Y (2003) J Org Chem 68:6274–8
Li S, Fuchino H, Kawahara N, Sekita S, Satake M (2002) J Nat Prod 65:262–266
Dean FM (1973) The Total Synthesis of Natural Products. ApSimon J Ed.: New York, vol. 1, pp. 513–525
Rozhkov RV, Larock RC J Org Chem submitted for publication
United States Food and Drug Administration, Chirality 4 (1992) 338–340
Saito H, Oishi H, Kitagaki S, Nakamura S, Anada M, Hashimoto S (2002) Org Lett 4:3887–90
Armstrong DW, DeMond W (1984) J Chromatogr Sci 22:411–415
Armstrong DW, Ward T, Beesley T (1986) Science 232:1132–1135
Armstrong DW, DeMond W, Czech B (1985) Anal Chem 57:481–484
Armstrong DW, Ward T, Czech A, Czech B, Bartsch R (1985) J Org Chem 50:5556–5559
Armstrong DW, Zukowski J (1994) J Chromatogr A 666:445–448
Armstrong DW, Chang L, Chang S, Wang X, Ibrahim H, Reid G, Beesley T (1997) Liq Chromatogr Rel Technol 20:3279–3295
Mitchell C, Desai M, McCulla R, Jenks W, Armstrong DW (2002) Chromatographia 56:127–135
Schumacher D, Mitchell C, Xiao T, Rozhkov RV, Larock RC, Armstrong DW (2003) J Chromatogr A 1011:37–47
Schumacher D, Mitchell C, Rozhkov RV, Larock RC, Armstrong DW (2004) J Liq Chromatogr Rel Technol in press
Han X, Yao T, Liu Y, Larock RC, Armstrong DW (2005) J Chromatogr A 1063:111–120
Narita S, Kitagawa T (1989) Anal Sci 5:361–2
Acknowledgments.
We would like to gratefully acknowledge the National Institutes of Health for funding this research (NIH RO1 GM53825-08),
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soukup, R., Rozhkov, R., Larock, R. et al. The Use of Cyclodextrin-Based LC Stationary Phases for the Separation of Chiral Dihydrobenzofuran Derivatives. Chroma 61, 219–224 (2005). https://doi.org/10.1365/s10337-005-0519-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-005-0519-6