Abstract
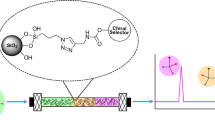
Two crystalline degradation products (CDP-1-M and CDP-1-m) are formed from vancomycin by hydrolytic loss of ammonia. The CDP are structurally similar to vancomycin, with two carboxyl groups, but are biologically inactive. In this work the CDP of vancomycin have been covalently linked to silica gel and their ability to resolve racemic mixtures, their stability, and their loading has been evaluated by HPLC. Elemental analysis and scanning electron microscopy were used to monitor the surface of this new stationary phase. Enantiomeric separation was achieved for some compounds that have not been previously been separated on other chiral stationary phases.
Similar content being viewed by others
References
Armstrong DW, Tang Y, Chem S, Zhou Y, Bagwill C, Chem JR (1994) Anal Chem 66:1473–1484
Tesarva E, Gilar M, Jegorov A, Uhroya M, Denyl Z (1997) Biomed Chromatogr 11:321–324
Ekborg KH, Kullman JP, Wang X, Gahm K, He L, Armstrong DW (1998) Chirality 10:627–660
Aboul-Enein HY, Ali I (2003) Macrocyclic glycopeptide antibiotics-based chiral stationary phase. In: Chiral separation by liquid chromatography and related technologies. Marcel Dekker, New York, Chap 4, pp 137–175
Aboul-Enein HY, Ali I (2000) Chromatographia 52:679–691
Hui F, Ekborg KH, Armstrong DW (2001) J Chromatogr A 906:91–103
Berthod A, Ling Xiao T, Liu Y, Jenks WS, Armstrong DW (2002) J Chromatogr A 955:53–69
Beesley TE (1999) Chirobiotic handbook, 3rd edn. Advance Separation Technologies
Subramanian G (2001) Chiral separation techniques. Wiley–VCH, Weinheim
Kamikawa K, Tachibana A, Sugimoto S, Uemura M (2001) Org Lett 3:2033–2036
Harris CM, Kopecka H, Harris TM (1983) J Am Chem Soc 105:6915–6922
Backes DW, Aboleneen HI, Simpson JA (1998) J Pharm Biomed Anal 16:1281–1287
Ghassempour A, Khalilian-Darbandi M, Salek-Asghari F (2001) Talanta 55:573–580
Berthod A, Nair UB, Bagwill C, Armstrong DW (1996) J Chromatogr A 43:1767–1782
Berthod A, Yu T, Kullman JP, Armstrong DW, Gasparrin F, D’Acquarica D, Misiti I, Carotti A (2000) J Chromatogr A 897:113–129
Berthod A, Liu Y, Bagwill C, Armstrong DW (1996) J Chromatogr A 731:123–137
Sun Q, Olesik SV (2000) J Chromatogr B 745:159–166
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghassempour, A., Abdollahpour, A., Tabar-Heydar, K. et al. Crystalline Degradation Products of Vancomycin as a New Chiral Stationary Phase for Liquid Chromatography. Chroma 61, 151–155 (2005). https://doi.org/10.1365/s10337-004-0489-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-004-0489-0