Abstract
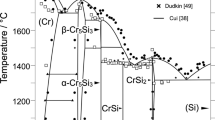
A Knudsen effusion method with mass-spectrometric analysis of gaseous phase has been applied to investigate the thermodynamic properties of the chromium phosphides (1341 to 1704 K) and Cr-P liquid alloys (1664 to 1819 K). Simultaneously, DSC has been used to measure heat capacities of chromium phosphides Cr3P and Cr12P7 in the temperature range of113 to 873 K. The entropies of formation of chromium phosphides calculated according to the second and third laws of thermodynamics agree within the limits of experimental error. The Gibbs energies of formation of the phosphides from solid Cr and P2 gas have been approximated with the following equations (in J/mol): AfG0(Cr3P) = −(244 112 ±2800) + (70.95 ±1.80)T ΔG0(Cr122P7) = −(1563 678 ±15 350) + (440.6 ±9.90)T Thermodynamic properties of liquid solutions have been described with the ideal associated-solution model assuming that CrP, Cr2P, Cr3P, and Cr3P2 complexes exist in the melt. The phase diagram computed with the help of the thermodynamic data agrees with the published information.
Similar content being viewed by others
Cited References
V.I. Pogorelii, V.K. Tagirov, E.K. Kasenas, V.Ya. Dashevskii, and B.I. Kashin,Reducing Processes in Production of Ferroalloys, Nauka, Moscow, 54 (1977) in Russian.
CE. Myers, G.A. Kisacky, and J.K. Klingert,J. Electrochem. Soc, 132, 236 (1985).
A.I. Zaitsev, M.A. Zemchenko, A.D. Litvina, and B.M. Mogutnov,Z. Metallkd., 84, 178 (1993).
A. I. Zaitsev, N. V. Korolyov, and B. M. Mogutnov,J. Chem. Thermodynamics, 23, 11 (1991).
A. I. Zaitsev, Zh. V. Dobrokhotova, A. D. Litvina, and B. M. Mogutnov,J. Chem. Soc, Faraday Trans., 91, 703 (1995).
L. V. Gurvich,Vestnik Akad. Nauk SSSR, 3, 54 (1983).
A. I. Zaitsev, M. A. Zemchenko, and B.M. Mogutnov,Russian J. Phys. Chem., 64, 3377 (1990).
A.I. Zaitsev, N.V. Korolyov, and B.M. Mogutnov,Teplophys. Vys. Temp. 27, 465 (1989) in Russian.
A.I. Zaitsev, N.V. Korolyov, and B.M. Mogutnov,High Temp. Sci., 28, 341 (1990).
A.I. Zaitsev,Zavod Lab., 56, 57 (1990) in Russian.
J. Drowart,Mass-spectrometry, J. Marsel, Ed., J. Stefen Inst., Ljubljana, 187–242 (1971).
I. Karakaya, and W.T. Tompson,Bull. Alloy Phase Diagrams, 9, 232 (1988).
J. B. Mann,Proc. Int. Conf. Mass Spectroscopy, T. Ogata and T. Hayakawa, Ed., University Park Press, Tokyo, 814 (1970).
D. Sh. Tsagareishvili, Dr. Sc. Thesis, Institut Metallurgii, Tbilisi, (1983) in Russian.
Zh.V. Dobrokhotova, A.I. Zaitsev, M.A. Zemchenko, A.D. Litvina, B.M. Mogutnov, and S.N. Yashchenko,J. Therm. Anal, 18, 1113 (1992).
A. I. Zaitsev, M. A. Zemchenko, and B. M. Mogutnov,Russ. J. Phys. Chem., 64, 1187 (1990).
M. Hansen and K. Anderko,Constitution of Binary Alloys, 2nd ed., McGraw-Hill, New York (1958).
F. Weibke, and G. Schrag,Z. Electrochem., 47, 222 (1941).
G. Lewis, and C. E. Myers,J. Phys. Chem., 67, 1289 (1963).
P. Spencer and O. A. Kubaschewski,Arch. Eisenhiittenwes., 49, 225 (1978).
J. Barin, O. Knacke, and O. Kubaschewski,Thermochemical Properties of Inorganic Substances, Supplement, Springer-Verlag, Berlin (1977).
R. Hultgren, P. D. Desai, D. T. Hawkins, et al.,Selected Values of the Thermodynamic Properties of the Elements, American Society for Metals, Metals Park, OH, 134 (1973).
G. R. Belton, and R. J. Fruechan,Metall. Trans., 2, 291 (1971).
H. G. Hadrys, M. G. Frohberg, J. Elliott, and C.H.P. Lupis,Metall. Trans., 1, 1867 (1970).
M.G. Frohberg, J. Elliott, and H.G. Hadrys,Arch. Eisenhiittenwes., 39, 587 (1968).
Y. E. Lee,Metall. Trans. B, 17, 777 (1986).
E. Schurmann, H.P. Kaiser, and U. Hensgen,Arch. Eisenhiittenwes., 52, 51 (1981).
M. Yamamoto, K. Yamada, L.L. Meschkov, and E. Kato,Tetsu-to-Hagané, 66, 2032 (1980).
J. Bookey,J. Iron Steel Inst., 172, 61 (1952).
H. Schenck, E. Steinmetz, and R. Gohlke,Arch. Eisenhuttenwes., 37, 775 (1966).
E.T. Turkdogan,Physical Chemistry of High Temperature Technology, Academic Press, London (1980).
R. Vogel and G. W. Kasten,Arch. Eisenhuttenwes., 12, 387 (1939).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zaitsev, A.I., Shelkova, N.E., Litvina, A.D. et al. Thermodynamic properties and phase equilibria in the Cr-P system. J Phase Equil. 19, 191–199 (1998). https://doi.org/10.1361/105497198770342201
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1361/105497198770342201