Abstract
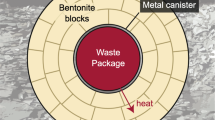
Excavated bentonite from two large iron-bentonite field experiments at Äspö Hard Rock Laboratory in Sweden was investigated with respect to iron redox chemistry and mineralogy. The iron redox chemistry was studied by Fe K-edge X-ray absorption near edge structure spectroscopy and the mineral phases were studied using X-ray diffraction. Bentonite is to be used as a buffer material in high-level radioactive waste repositories to protect the waste containers from their surroundings. Montmorillonite, which is responsible for the sealing properties in the bentonite, is susceptible to redox reactions. A change in the montmorillonite iron redox chemistry may affect its layer charge and hence its properties. The experiments included are the first Alternative Buffer Material test (ABM1) and the Temperature Buffer Test (TBT). The clays were heated to a maximum of ~130°C (ABM1) or ~150°C (TBT) for 2.5 and 7 y, respectively. In the central part of the compacted clay blocks was placed an iron heater and the distance from the heater to the rock was ~10 cm (ABM1) and ~50 cm (TBT), respectively. Eleven different clay materials were included in the ABM1 experiment and five were analyzed here. In the ABM1 experiment, the Fe(II)/Fe(III) ratio was increased in several samples from the vicinity of the heater. Kinetic data were collected and showed that most of the Fe(II)-rich samples oxidized rapidly when exposed to atmospheric oxygen. In the TBT experiment the corrosion products were dominated by Fe(III) and no significant increase in Fe(II) was seen. In ABM1, reducing conditions were achieved, at least in parts of the experiment; in TBT, reducing conditions were not achieved. The difference was attributed to the larger scale of the TBT experiment, providing more oxygen after the installation, and to the longer time taken for water saturation; oxidation of the samples during excavation cannot be ruled out. Minor changes in the bentonite mineral phases were found in some cases where direct contact was made with the iron heater but no significant impact on the bentonite performance in high-level radioactive waste applications was expected as a result.
Similar content being viewed by others
References
Ardizzone, S. and Formaro, L. (1982) Temperature induced phase transformation of metastable Fe(OH)3 in the presence of ferrous ions. Materials Chemistry and Physics, 8, 125–133.
Akesson, M., Olsson, S., Dueck, A., Nilsson, U., and Karnland. O. (2012) TBT — Hydro-mechanical and chemical/miner-alogical characterizations. SKB Report, P-12-06, SKB, Stockholm.
Bath, A. and Hermansson, H.-P. (2009) Biogeochemistry of redox at repository depth and implications for the canister. Swedish Radiation Safety Authority, Report 2009: 28.
Carlson, L., Karnland, O., Oversby, V.M., Rance, A.P., Smart, N.R., Snellman, M., Vähänen, M., and Werme, L.O. (2007) Experimental studies of the interactions between anaerobi-cally corroding iron and bentonite. Physics and Chemistry of the Earth, 32, 334–345.
Carlson, S., Clausén, M., Gridneva, L., Sommarin, B., and Svensson, C. (2006) XAFS experiments at beamline 1811, MAX-lab synchrotron source, Sweden. Journal of Synchrotron Radiation, 13, 359–364.
Cerenius, Y., Stahl, K., Svensson, L.A., Ursby, T., Oskarsson, A., Albertsson, J., and Liljas, A., (2000) The crystallography beamline 1711 at MAX II. Journal of Synchrotron Radiation, 7, 203.
Charpentiera, D., Devineau, K., Mosser-Ruck, R., Cathelineau, M., and Villiéras, F. (2006) Bentonite-iron interactions under alkaline condition: An experimental approach. Applied Clay Science, 32, 1–13.
Crank, J. (1980) The Mathematics of Diffusion, 2nd edition, Clarendon Press, Oxford.
Dohrmann, R., Olsson, S., Kaufhold, S., and Sellin, P. (2013) Mineralogical investigations of the first package of the alternative buffer material test II. Exchangeable cation population rearrangement. Clay Minerals, 48, 215–233.
Galoisy, L., Calas, G., and Arrio, M.A. (2001) High-resolution XANES spectra of iron in minerals and glasses: structural information from the pre-edge region. Chemical Geology, 174, 307–319.
Gaucher, E.C., Tournassat, C., Pearson, F.J., Blanc P., Crouzet, C., Lerouge, C., and Altmann, S. (2009) A robust model for pore-water chemistry of clayrock. Geochimica et Cosmochimica Acta, 73, 6470–6487.
Grandia, F., Domènech, C., Arcos, D., and Duro, L. (2006) Assessment of the oxygen consumption in the backfill. Geochemical modelling in a saturated backfill. SKB Report, R-06-106, SKB, Stockholm.
Grenthe, L., Stumm, W., Laaksuharju, M., Nilsson, A.-C, and Wikberg, P. (1992) Redox potentials and redox reactions in deep groundwater systems. Chemical Geology, 98, 131–150.
Guillaume, D., Neaman, A., Cathelineau, M., Mosser-Ruck, R., Peiffert, C., Abdelmoula, M., Dubessy, J., Villiéras, F., and Michau, N. (2004) Experimental study of the transformation of smectite at 80 and 300°C in the presence of Fe oxides. Clay Minerals, 39, 17–34.
Jodin-Caumon, M.C., Mosser-Ruck, R., Rousset, D., Randi, A., Cathelineau, M., and Michau, N. (2010) Effect of a thermal gradient of iron-clay interactions. Clays and Clay Minerals, 58, 667–681.
Kaufhold, S., Dohrmann, R., Sandén, T., Sellin, P., and Svensson, D. (2013) Mineralogical investigations of the alternative buffer material test — I. Alteration of bentonites. Clay Minerals, 48, 199–213.
Kumpulainen, S., Kiviranta, L., Carlsson, T., Muurinen, A., Svensson, D., Sasamoto, H., and Wersin, P. (2010) Long-term alteration of bentonite in the presence of metallic iron. SKB Report, R-10-52, SKB, Stockholm.
Kwiatek, W.M., Galka, M., Hanson, A.L., Paluszkiewicz, C., and Cichocki, T. (2001) XANES as a tool for iron oxidation state determination in tissues. Journal of Alloys and Compounds, 328, 276–282.
Lantenois, S., Lanson, B., Muller, F., Bauer, A., Jullien, M., and Plançon, A. (2005) Experimental study of smectite interaction with metal Fe at low temperature: 1. Smectite destabilization. Clays and Clay Minerals, 53, 597–612.
Martin, F.A., Bataillon, C., and Schlegel, M.L. (2008) Corrosion of iron and low alloyed steel within a water saturated brick of clay under anaerobic deep geological disposal conditions: An integrated experiment. Journal of Nuclear Materials, 379, 80–90.
Mehra, O.P. and Jackson, M.L. (1958) Iron oxide removal from soils and clays by a dithionite citrate system buffered with sodium bicarbonate. Clays and Clay Minerals, 7, 317–327.
Meier, L.P. and Kahr, G. (1999) Determination of the cation exchange capacity (CEC) of clay minerals using complexes of copper(II) ion with triethylenetetramine and tetraethylenepentamine. Clays and Clay Minerals, 47, 386–388.
Mosser-Ruck, R., Cathelineau, M., Guillaume, D., Charpentiera, D., Rousset, D., Barres, O., and Michaue, N. (2010) Effects of temperature, pH, and iron/clay and liquid/ clay ratios on experimental conversion of dioctahedral smectite to berthierine, chlorite, vermiculite, or saponite. Clays and Clay Minerals, 58, 280–291.
O’Day, P.A., Rivera Jr., N., Root, R., and Carroll S.A. (2004) X-ray absorption spectroscopic study of Fe reference compounds for the analysis of natural sediments. American Mineralogist, 89, 572–585.
Ogawa, M., Sato, T., Takahashi, N., and Tanaka, M. (1991) Synthetic stevensite and process for preparation thereof. U.S. Patent 5,004,716 A, filed April 2, 1990, and published April 2, 1991.
Paris, E., Mottana, A., and Mattias, P. (1991) Iron environment in a montmorillonite from Gola del Furlo (Marche, Italy). A synchrotron radiation XANES and a Mössbauer study. Mineralogy and Petrology, 45, 105–117.
Pentráková, L., Su, K., Pentrák, M., and Stucki, J. W. (2013) A review of microbial redox interactions with structural Fe in clay minerals. Clay Minerals, 48, 543–560.
Perronnet, M., Jullien, M., Villiéras, F., Raynal, J., Bonnin, D., and Bruno, G. (2008) Evidence of a critical content in Fe(0) on FoCa7 bentonite reactivity at 80°C. Applied Clay Science, 38, 187–202.
Quartieri, S., Riccardi, M.P., Messiga, B., and Boscherini, F. (2005) The ancient glass production of the medieval Val Gargassa glasshouse: Fe and Mn XANES study. Journal of Non-Crystalline Solids, 351, 3013–3022.
Ronov, A.B. and Yaroshevsky, A.A. (1969) Chemical composition of the Earth’s crust. Pp 37–57 in: The Earth’s Crust and Upper Mantle (I. Hart, editor). Geophysical Monograph, 13, American Geophysical Union, Washington, DC.
Sandén, T., Goudarzi, R., Combarieu, M. Åkesson, M., and Hökmark, H. (2007) Temperature buffer test — design, instrumentation and measurements. Physics and Chemistry of the Earth, 32, 77–92.
SKB (2001) O2 depletion in granitic media. The REX project. SKB Technical Report, TR-01-05, SKB, Stockholm.
SKB (2007) RD & D Programme. Programme for research, development and demonstration of methods for the management and disposal of nuclear waste. SKB Technical Report, TR-07-12, SKB, Stockholm.
Stucki, J.W., Lee, K., Zhang, L., and Larson, R.A. (2002) Effects of iron oxidation state on the surface and structural properties of smectites. Pure and Applied Chemistry, 74, 2081–2094.
Svensson, D. (2013) Early observations in a large scale 61/2 year iron-bentonite field experiment (ABM2) at Äspö hard rock laboratory, Sweden. Conference abstract, 50th annual meeting of The Clay Minerals Society. October 6–10, Urbana-Champaign, Illinois, USA.
Svensson, D., Eng, A., and Sellin, P. (2007) Alternative buffer material experiment. Conference abstract, 3rd International meeting, Clays in natural & engineered barriers for radio-active waste confinement, September 17–18, Lille, France.
Svensson, D., Sandén, T., Kaufhold, S., and Sellin, P. (2010) Alternative buffer material experiment — experimental concept and progress. Conference abstract, 4th International meeting, Clays in natural & engineered barriers for radioactive waste confinement. March 29–April 1, Nantes, France.
Svensson, D., Dueck, A., Nilsson, U., Olsson, S., Sandén, T., Eriksson, S., Jägervall, S., and Hansen, S. (2011) Alternative Buffer Material. Status of the ongoing laboratory investigation of reference materials and test package 1. SKB Technical Report, TR-11-06, SKB, Stockholm
Wersin, P., Spahiu, K., and Bruno, J. (1994) Time evolution of dissolved oxygen and redox conditions in a HLW repository. SKB Technical Report, TR-94-02, SKB, Stockholm.
Wersin, P., Birgersson, M., Olsson, S., Karnland, O., and Snellman, M. (2008) Impact of corrosion-derived iron on the bentonite buffer within the KBS-3H disposal concept. The Olkiluoto site as case study. SKB Report, R-08-34, SKB, Stockholm.
White, A.F. and Yee, A. (1985) Aqueous oxidation-reduction kinetics associated with coupled electron-cation transfer from iron-containing silicates at 25°C. Geochimica et Cosmochimica Acta, 49, 1263–1275.
Wilke, M., Farges, F., Petit, P.-E., Brown Jr., G.E., and Martin, F. (2001) Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. American Mineralogist, 86, 714–730.
Wilke, M., Partzsch, G.M., Bernhardt, R., and Lattard, D. (2005) Determination of the iron oxidation state in basaltic glasses using XANES at the K-edge. Chemical Geology, 220, 143–161.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svensson, P.D., Hansen, S. Redox Chemistry in Two Iron-Bentonite Field Experiments at Äspö Hard Rock Laboratory, Sweden: An XRD and Fe K-Edge Xanes Study. Clays Clay Miner. 61, 566–579 (2013). https://doi.org/10.1346/CCMN.2013.0610609
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2013.0610609