Abstract
Layered double hydroxides (LDHs) are layered ion exchangers, with a large surface-charge density, which react easily with organic anions. Various types of organics are rapidly substituted in the interlayer space of inorganic precursor LDHs. ZnAl-LDHs were intercalated with 1- to 19-carbon monocarboxylic acid anions by anion exchange of NO3-saturated LDH precursor phases in order to study the dependence of exchange reactions on synthesis parameters (temperature, pH, and interlayer anion). The carboxylic acid anion-LDHs synthesized were characterized using X-ray diffraction, infrared spectroscopy, thermal analysis, scanning electron microscopy, chemical analysis, and N2 adsorption. Carboxylic anion quantities in excess of the LDH anion exchange capacity easily replaced exchangeable nitrate anions at moderate pH. The intercalated LDH interlayer space depended on the alkyl chain length and orientation (inclination angle) of thecarboxylic-acid anion. Thelatticeparameter c0 ranged from 3.4 to 13.5 nm, but the a0 lattice parameter remained constant at 0.31 nm. Crystallographic analyses indicated a monomolecular arrangement of intercalated short-chain fatty-acid anions. At pH < 7, intercalated long-chain carboxylates showed a preferred bimolecular interlayer orientation. Carboxylic-acid anion exchange with LDHs at pH 7 resulted in the formation of two different sets of basal spacings, which indicated the coexistence of LDHs intercalated with monomolecular and bimolecular arrangements of interlayer carboxylic compounds.
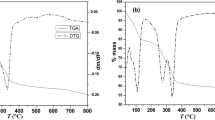
Thermal treatment of the carboxylic acid anion-intercalated LDHs indicated stability up to ~140ºC. The release of interlayer water led to distortion of the crystallographic units and resulted in smaller basal spacings without collapse of the layered structure. Heat treatment re-oriented alkyl-chain carbon carboxylates (with >10 carbons) to a more upright interlayer position.
Similar content being viewed by others
References
Anbarasan, R., Lee, W.D., and Im, S.S. (2005) Adsorption and intercalation of anionic surfactants onto layered double hydroxides-XRD study. Bulletin of Materials Science, 28, 145–149.
Bish, D.L. (1980) Anion-exchange in takovite: applications to the other hydroxide minerals. Bulletin of Mineralogy, 103, 170–175.
Carlino, S. (1997) Theintercalation of carboxylic acids into layered double hydroxides: a critical evaluation and review of the different methods. Solid State Ionics, 98, 73–84.
Cavani, F., Trifiro, F., and Vaccari, A. (1991) Hydrotalcite-type anionic clays: Preparation, properties and applications. Catalysis Today, 11, 173–301.
Choudhary, V.R., Dumbre, D.K., Narkhede, V.S., and Jana, S.K. (2003) Solvent-free selective oxidation of benzyl alcohol and benzaldehyde by tert-butyl hydroperoxide using MnO4-exchanged Mg-Al-hydrotalcite catalysts. Catalysis Letters, 86, 229–233.
Cooper, M.A. and Hawthorne, F.C. (1996) The crystal structureof shigaite, [AlMn22+ (OH)6]3(SO4)2 Na(H2O)6(H2O)6, a hydrotalcite-group mineral. The Canadian Mineralogist, 34, 91–97.
Hansen, B., Curtius, H., and Odoj, R. (2009) Synthesis of a Mg-Cd-Al layered double hydroxide and sorption of selenium. Clays and Clay Minerals, 57, 330–337.
Itoh, T., Ohta, N., Shichi, T., Yui, T., and Takagi, K. (2003) The self-assembling properties of stearate ions in hydro-talciteclay composites. Langmuir, 19, 9120–9126.
Khan, A.I. and O’Hare, D. (2002) Intercalation chemistry of layered double hydroxides: recent developments and applications. Journal of Materials Chemistry, 12, 3191–3198.
Kloprogge, J.T., Wharton, D., Hickey, L., and Frost, R.L. (2002) Infrared and Raman study of interlayer anions CO3−2, NO3, SO42 and ClO4− in Mg/Al-hydrotalcite. American Mineralogist, 87, 623–629.
Kopka, H., Beneke, K., and Lagaly, G. (1988) Anionic surfactants between double metal hydroxide layers. Journal of Colloid and Interface Science, 123, 427–436.
Lagaly, G. (1981) Inorganic layer compounds. Naturwissenschaften, 68, 82–88.
Meyn, M., Beneke, K., and Lagaly, G. (1990) Anion-exchange reactions of layered double hydroxides. Inorganic Chemistry, 29, 5201–5207.
Nhlapo, N., Motumi, T., Landman, E., Verryn, A.M.C., and Focke, W.W. (2008) Surfactant-assisted fatty intercalation of layered double hydroxides. Journal of Materials Science, 43, 1033–1043.
Ogawa, M. and Kaiho, H. (2002) Homogeneous precipitation of uniform hydrotalciteparticles. Langmuir, 18, 4240–4242.
Poellmann, H. (2007) Immobilisierung von Schadstoffen durch Speichermineralbildung. Shaker-Verlag, Achen, Germany, pp. 130–150.
Prasanna, S.V., Radha, A.V., Kamath, P.V., and Kannan, S. (2009) Bromide-ion distribution in the interlayer of the layered double hydroxides of Zn and Al: Observation of positional Disorder. Clays and Clay Minerals, 57, 82–92.
Reichle, W.T. (1986) Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ionics, 22, 135–141.
Reinholdt, M.X. and Kirkpatrick, R.J. (2006) Experimental investigations of amino acid-layered double hydroxide complexes: glutamate-hydrotalcite. Chemistry of Materials, 18, 2567–2576.
Roy, A., Forano, C. and Besse, J.P. (2001) Layered double hydroxides: Synthesis and postsynthesis modifications. Pp. 1–38 in: Layered Double Hydroxides: Present and Future (V. Rives, editor). Nova Science Publishers, Inc., New York.
Wypych, F., Arı´zaga, G.G.C., and da Costa Gardolinski, J.E.F. (2005) Intercalation and functionalization of zinc hydroxide nitratewith mono- and dicarboxylic acids. Journal of Colloid and Interface Science, 283, 130–138.
Xu, Z.P. and Bratermann, P.S. (2007) Competitive Intercalation of Sulfonates into Layered Double Hydroxides (LDHs): the Key Roleof Hydrophobic Interactions. Journal of Physical Chemistry C, 111, 4021–4026.
Yang, L., Sharivari, Z., Liu, P.K.T., Sahimi, M., and Tsotsis, T.T. (2005) Removal of trace levels of arsenic and selenium from aqueous solutions by calcined and uncalcined layered doublehydroxides (LDH). Industrial & Engineering Chemical Research, 44, 6804–6815.
Zhao, H. and Vance, G.F. (1998) Selectivity and molecular sieving effects of organic compounds by a ß-cyclodextrin-pillared layered double hydroxide. Clays and Clay Minerals, 46, 712–718.
Zhu, J., Yuan, P., He, H., Frost, R., Tao, Q., Shen, W., and Bostrom, T. (2008) In situ synthesis of surfactant/silane-modified hydrotalcites. Journal of Colloid and Interface Science, 319, 498–504.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuehn, T., Poellmann, H. Synthesis and Characterization of Zn-Al Layered Double Hydroxides Intercalated With 1- to 19-Carbon Carboxylic Acid Anions. Clays Clay Miner. 58, 596–605 (2010). https://doi.org/10.1346/CCMN.2010.0580502
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2010.0580502