Abstract
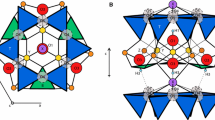
In order to improve our understanding of how the goethite crystal structure is affected by the incorporation of metals (and by variations in the amount of the incorporation), and to review any possible synergistic and antagonistic effects of co-metals, the present investigation focused on the incorporation of multiple (di-, tri-, and tetra-) metals, i.e. Cr, Zn, Cd, and Pb, in the goethite crystallographic structure. A series of single- and multi-metal M-Cr/Zn/Cd/Pb-substituted goethites with M/(M+Fe) molar ratios = 0.10 were prepared. The general sequence of metal entry in single-metal substituted goethites was Zn = Cr > Cd > Pb and in multi-metal-substituted goethites was Zn > Cr ⩾ Cd > Pb. Simultaneous incorporation of Cr, Zn, Cd, and Pb up to 10.5 mole % was achieved in goethite. Synchrotron X-ray diffraction and extended X-ray absorption fine structure (EXAFS) techniques were employed to assess the structural characteristics of the synthesis products. Rietveld refinement of XRD data showed small changes in unit-cell parameters and Fe/M-Fe/M distances due to M substitution(s). A typical goethite-like crystalline structure remained intact, however. The unit-cell parameters were mutually, linearly correlated, though Fe/M-Fe/M distances were not, indicating that complex changes occurred at the local scale. In single-metal substituted goethites, incorporation of Cr reduced the unit-cell volume by 0.13% while that of Zn, Cd, and Pb increased it by 1.09, 3.58, and 0.56%, respectively. The changes in multi-metal-substituted goethites appeared to be the complex combination of that of the individually incorporated metals. The X-ray absorption near edge structure study of Pb-substituted goethites showed that the majority of associated Pb was Pb2+, while Pb4+ was preferred over Pb2+ in the bulk structure. Measurements by EXAFS at the Fe K-edge indicated that the Fe polyhedra contracted in the presence of Cd2+ and Pb2+, providing room for the substitution of larger cations. Measurements by EXAFS at the Cr and Cd K-edges indicated symmetric Cr/Cd polyhedra with single Cr/Cd-O distances and, at Fe and Zn K-edges and the Pb LIII-edge, indicated asymmetric polyhedra with two sets of Fe/Zn/Pb-O distances. The Zn octahedra were possibly Zn(OH)4O2, which enlarged the metal-metal corner-sharing distance to 3.86 Å. This configuration of ligands around the Zn2+ cation might occur to balance local charges. Symmetric polyhedra appeared to reduce steric strains in the structure, compared to the asymmetric polyhedra. The result was that Cr enhanced the incorporation of Zn, Cd, and Pb, while the converse was true for Zn.
Similar content being viewed by others
References
Alvarez, M., Rueda, E.H., and Sileo, E.E. (2007) Simultaneous incorporation of Mn and Al in the goethite structure. Geochimica et Cosmochimica Acta, 71, 1009–1020.
Carvalho-E-Silva, M.L., Ramos, A.Y., Tolentino, H.C.N., Enzweiler, J., Netto, S.M., and Alves, M. (2003) Incorporation of Ni into natural goethite: An investigation by X-ray absorption spectroscopy. American Mineralogist, 88, 876–882.
Cornell, R.M. (1988) The influence of some divalent cations on the transformation of ferrihydrite to more crystalline products. Clay Minerals, 23, 329–332.
Cornell, R.M. (1991) Simultaneous incorporation of Mn, Ni and Co in the goethite (alpha-FeOOH) structure. Clay Minerals, 26, 427–430.
Cornell, R.M. and Schwertmann, U. (2003) The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley-VCH Verlag Gmbh & Co. KGaA Weinheim, Germany.
Ellis, P.J. and Freeman, H.C. (1995) XFIT — an interactive EXAFS analysis program. Journal of Synchrotron Radiation, 2, 190–195.
Ford, R.G., Bertsch, P.M., and Farley, K.J. (1997) Changes in transition and heavy metal partitioning during hydrous iron oxide aging. Environmental Science & Technology, 31, 2028–2033.
Gerth, J. (1990) Unit cell dimensions of pure and trace metal-associated goethites. Geochimica et Cosmochimia Acta, 54, 363–371.
Gräfe, M., Singh, B., and Balasubramaniam, M. (2007) Surface speciation of Cd(II) and Pb (II) on kaolinite by XAFS spectroscopy. Journal of Colloid and Interface Science, 315, 21–32.
Gräfe, M., Mustafa, G., Singh, B., and Kookana, R.S. (2008) Solid phase partitioning of cadmium (Cd2+) in goethite media. Pp. 187–204 in: Adsorption of Metals by Geomedia II (M.O. Barnett and D.B. Kent, editors). Developments in Earth and Environmental Sciences, 7. Elsevier, Amsterdam.
Gualtieri, A. and Venturelli, P. (1999) In situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction. American Mineralogist, 84, 895–204.
Hunter, B.A. (1997) Rietica for Windows v. 1.7.7. ANSTO, Sydney, Australia.
Huynh, T., Tong, A.R., Singh, B., and Kennedy, B.J. (2003) Cd-substituted goethites — A structural investigation by synchrotron X-ray diffraction. Clays and Clay Minerals, 51, 397–402.
Krehula, S. and Music, S. (2007) The influence of Cd-dopent on the properties of alpha-FeOOH and alpha-Fe2O3 particles precipitated in highly alkaline media. Journal of Alloys and Compounds, 431, 56–64.
Krehula, S., Music, S., Skoko, Z., and Popovic, S. (2006) The influence of Zn-dopant on the precipitation of alpha-FeOOH in highly alkaline media. Journal of Alloys and Compounds, 420, 260–268.
Manceau, A. and Drits, V.A. (1993) Local-structure of ferrihydrite and feroxyhite by EXAFS spectroscopy. Clay Minerals, 28, 165–184.
Manceau, A., Schlegel, M.L., Musso, M., Sole, V.A., Gauthier, C., Petit, P.E., and Trolard, F. (2000) Crystal chemistry of trace elements in natural and synthetic goethite. Geochimica et Cosmochimica Acta, 64, 3643–3661.
Martinez, C.E. and McBride, M.B. (1998) Coprecipitates of Cd, Cu, Pb and Zn in iron oxides: Solid phase transformation and metal solubility after aging and thermal treatment. Clays and Clay Minerals, 46, 537–545.
Rehr, J.J., Mustre de Leon, J., Zabinski, S.I., and Albers, R.C., 1991. (1991) Theoretical X-ray absorption fine structure standards. Journal of the American Chemical Society, 113, 5135–5145.
Ressler, T. (1998) WinXAS: a program for X-ray absorption spectroscopy data analysis under MS-Windows. Journal of Synchrotron Radiation, 5, 118–122.
Rietveld, H.M. (1969) A profile refinement method for nuclear and magnetic structures. Journal of Applied Crystallography, 2, 65–71.
Schwertmann, U., Gasser, U., and Sticher, H. (1989) Chromium-for-iron substitution in synthetic goethites. Geochimica et Cosmochimica Acta, 53, 1293–1297.
Sileo, E.E., Ramos, A.Y., Magaz, G.E., and Blesa, M.A. (2004) Long-range vs. short-range ordering in synthetic Cr-substituted goethites. Geochimica et Cosmochimica Acta, 68, 3053–3063.
Singh, B. (2001) Heavy metals in soils: sources, chemical reactions and forms. Pp. 77–93 in: Environmental Geotechnics (D. Smith, S. Fityus, and M. Allman, editors). Proceedings of the 2nd Australia and New Zealand Conference on Environmental Geotechnics — Geoenvironment 2001. Australian Geochemical Society, Newcastle, Australia.
Singh, B. and Gilkes, R.J. (1992) Properties and distribution of iron oxides and their association with minor elements in the soils of south-western Australia. Journal of Soil Science, 43, 77–98.
Singh, B., Harris, P.L., and Wilson, M.J. (1997) Geochemistry of Acid Mine waters and the role of micro-organisms in such environments: a review. Advances in Geoecology, 30, 159–192.
Singh, B., Sherman, D.M., Gilkes, R.J., Wells, M.A., and Mosselmans, J.F.W. (2002) Incorporation of Cr, Mn and Ni into goethite (α-FeOOH): mechanism from extended X-ray absorption fine structure spectroscopy. Clay Minerals, 37, 639–649.
Szytula, A., Burewicz, A., Dimitrijevic, Z., Krasnicki, S., Rzany, H., Todorovic, J., Wanic, A., and Wolski, W. (1968) Neutron diffraction studies of α-FeOOH. Physica Status Solidi, 26, 429–434.
Wedepohl, K.H. (1969) Handbook of Geochemistry. Springer, Berlin, Heidelberg, New York.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, N., Gräfe, M., Singh, B. et al. Simultaneous incorporation of Cr, Zn, Cd, and Pb in the goethite structure. Clays Clay Miner. 57, 234–250 (2009). https://doi.org/10.1346/CCMN.2009.0570210
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2009.0570210