Abstract
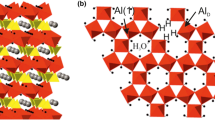
The configuration of hydroxyl groups around the octahedral cations of 2:1 phyllosilicate minerals has long been an important question in clay science. In the present study, 27Al multiple quantum (MQ) magic angle spinning nuclear magnetic resonance (MAS NMR) was applied to the local structural analysis of octahedral Al positions in a purified Na-montmorillonite. Three octahedral Al sites ([6]Ala, [6]Alb, and [6]Alc) are distinguished by 27Al 5QMAS NMR, whereas these sites are not differentiated by 27Al MAS and 3QMAS NMR. The isotropic chemical shift (δcs) and the quadrupolar product (PQ) were estimated to be 5.8 ppm and 2.6 MHz for [6]Ala, 6.2 ppm and 3.0 MHz for [6]Alb, and 6.7 ppm and 3.7 MHz for [6]Alc, respectively. The three Al sites originated from geometric isomers with cis and trans structures, which have mutually different configurations of the OH groups around the central Al3+ ions. From the view point of symmetry for the OH groups, [6]Ala and [6]Alb in the upfield region were assigned to cis sites, and [6]Alc in the downfield region was assigned to a trans site. The occurrence of multiple Al sites implies that Na-montmorillonite used in the present study has cis-vacant structure in the octahedral sheet. This is a reasonable insight, supported by the chemical composition and the differential thermal analysis data of the Na-montmorillonite.
Similar content being viewed by others
References
Abollino, O., Aceto, M., Malandrino, M., Sarzanini, C., and Mentasti, E. (2003) Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Research, 37, 1619–1627.
Amoureux, J.P., Huguenerd, C., Engelke, F., and Taulelle, F. (2002) Unified representation of MQMAS and STMAS NMR of half-integer quadrupolar nuclei. Chemical Physics Letters, 356, 497–504.
Bardy, M., Bonhomme, C., Fritsch, E., Maquet, J., Hajjar, R., Allard, T., Derenne, S., and Calas, G. (2007) Al speciation in tropical podzols of the upper Amazon Basin: A solid-state 27Al MAS and MQMAS NMR study. Geochimica et Cosmochimica Acta, 71, 3211–3222.
Benna, M., Kbir-Ariguib, N., Clinard, C., and Bergaya, F. (2001) Static filtration of purified sodium bentonite clay suspensions. Effect of clay content. Applied Clay Science, 19, 103–120.
Brevard, C. and Granger, P. (1983) Ruthenium NMR spectroscopy: a promising structural and analytical tool. General trends and applicability to organometallic and inorganic chemistry. Inorganic Chemistry, 22, 532–535.
Cong, X. and Kirkpatrick, R.J. (1996) 29Si MAS NMR Study of the Structure of Calcium Silicate Hydrate. Advanced Cement Based Materials, 3, 144–156.
Cuadros, J. (2002) Structural insights from the study of Cs-exchanged smectites submitted to wetting-and-drying cycles. Clay Minerals, 37, 473–486.
Drits, V.A., Besson, G., and Mueller, F. (1995) An improved model for structural transformation of heat-treated aluminous dioctahedral 2:1 layer silicates. Clays and Clay Minerals, 43, 718–731.
Drits, V.A., McCarty, D.K., and Zviagina, B.B. (2006) Crystal-chemical factors responsible for the distribution of octahedral cations over trans and cis sites indioctahedral 2:1 layer silicates. Clays and Clay Minerals, 54, 131–152.
Fitzgerald, J.J., Hamza, A.I., Bronnimann, C.E., and Dec, S.F. (1995) Studies of the solid/solution ‘interfacial’ delamination of kaolinite in HCL(aq) using 1H CRAMPS and SP/MAS 29Si NMR spectroscopy. Journal of the American Chemical Society, 119, 7105–7113.
Frydman, L. and Harwood, J.S. (1995) Isotropic spectra of half-integer quadrupolar spins from bidimensional magic-angle spinning NMR. Journal of the American Chemical Society, 117, 5367–5368.
Gore, K.U., Abraham, A., Hegde, H., Kumar, R., Amoureux, J.P., and Ganapathy, S. (2002) 29Si, and 27Al MAS/3Q-MAS NMR Studies of High Silica USY Zeolites. Journal of Physical Chemistry B, 106, 6115–6120.
Hedley, C.B., Yuan, G., and Theng, B.K.G. (2007) Thermal analysis of montmorillonites modified with quaternary phosphonium and ammonium surfactants. Applied Clay Science, 35, 180–188.
Juranić, N., Ćelap, M.B., Vućelić, D., Malinar, M.J., and Radivoja, P.N. (1977) The 13C and 59Co nuclear magnetic resonance study of mixed Co(III) complexes containing glycinato ligand. Inorganiea Chimiea Acta, 25, 229–232.
Koerner, H., Hampton, E., Dean, D., Turgut, Z., Drummy, L., Mirau, P., and Vaia, R. (2005) Generating triaxial reinforced epoxy/montmorillonite nanocomposites with uniaxial magnetic fields. Chemistry of Materials, 17, 1990–1996.
Komine, H. (2004) Simplified evaluation of swelling characteristics of bentonites. Engineering Geology, 71, 265–279.
Laszlo, P. (editor) (1983) NMR of Newly Accessible Nuclei. chapter 9. Vol, 2. Academic Press, London.
Lippmaa, E., Mägi, M., Samson, A., Engelhardt, G., and Grimmer, A.R. (1980) Structural studies of silicates by solid-state high-resolutionsilicon-29 NMR. Journal of the American Chemical Society, 102, 4889–4803.
Lippmaa, E., Samson, A., and Magi, M. (1986) High-resolution aluminum-27 NMR of aluminosilicates. Journal of the American Chemical Society, 108, 1730–1735.
Mann, B.E. (1991) Transition Metal NMR. Elsevier, New York, 177 pp.
Mooney, R.W., Keenan, A.G., and Wood, L.A. (1952) Adsorption of water vapor by montmorillonite. II. Effect of exchangeable ions and lattice swelling as measured by X-ray diffraction. Journal of the American Chemical Society, 74, 1271–1374.
Ohkubo, T., Kanehashi, K., Saito, K., and Ikeda, Y. (2003) Observationof two 4-coordinated Al sites in montmorillonite using high magnetic field strength 27Al MQMAS NMR. Clays and Clay Minerals, 51, 513–518.
Rochon, R.D., and Buculei, V. (2004) Multinuclear NMR study and crystal structure of complexes of the types cis and trans-Pt(amine)2I2. Inorganica Chimica Acta, 357, 2218–2230.
Sato, H. (2005) Effects of the orientation of smectite particles and ionic strength on diffusion and activation enthalpies of I− and Cs+ ions in compacted smectite. Applied Clay Science, 29, 267–281.
Steel, P.J., Ahousse, F.L., Lerner, D., and Marzin, C. (1983) New ruthenium(II) complexes with pyridylpyrazole ligands. Photosubstitutionand 1H, 13C, and 99Ru NMR structural studies. Inorganic Chemistry, 22, 1488–1493.
Takahashi, T., Ohkubo, T., and Ikeda, Y. (2006) Montmorillonite alignment induced by magnetic field: Evidence based on the diffusion anisotropy of water molecules. Journal of Colloid and Interface Science, 299, 198–203.
Takahashi, T., Ohkubo, T., Suzuki, K., and Ikeda, Y. (2007) High resolution solid state NMR study on dissolution and alteration of Na-montmorillonite under highly alkaline conditions. Microporous and Mesoporous Materials, 106, 284–297.
Tsipursky, S.I. and Drits, V.A. (1984) The distribution of octahedral cations in the 2:1 layers of dioctahedral smectites. Clay Minerals, 19, 177–193.
Uno, Y., Sasaki, T., and Tatematsu T. (1992) Dehydration process of smectites under controlled steam pressure. Nendo Kagaku, 32, 129–138.
Xue, S. and Pinnavaia, T.J. (2007) Overview of clay-based polymer nanocomposites (CPN): Pp. 1–24 in: Clay-based Polymer Nanocomposites (CPN) (K.A. Carrado and F. Bergaya editors). CMS Workshop Lectures, The Clay Minerals Society, Chantilly, VA, USA.
Yamasaki, A., Yajima, F., and Fujiwara, S. (1968) Nuclear magnetic resonance studies on cobalt complexes. I. Cobalt-59 nuclear-magnetic resonance spectra of cobalt (III) complexes. Inorganica Chimica Acta, 2, 39–42.
Zazzi, Å., Hirsch, T.K., Lenova, E., Kaikkonen, A., Grins, J., Annersten, H., and Eden, M. (2006) Structural investigations of natural and synthetic chlorite minerals by X-ray diffraction, Mössbauer spectroscopy and solid-state nuclear magnetic resonance. Clays and Clay Minerals, 54, 252–265.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, T., Kanehashi, K. & Saito, K. First evidence of multiple octahedral Al sites in Na-montmorillonite by 27Al multiple quantum MAS NMR. Clays Clay Miner. 56, 520–525 (2008). https://doi.org/10.1346/CCMN.2008.0560505
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2008.0560505