Abstract
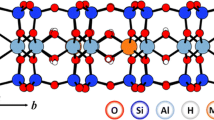
The objective of the study was to contribute to the understanding of the influence of the structure and the 2:1 layer dimension of smectites on cation exchange capacity (CEC) reduction and the hydration behavior of Li-saturated smectites after heating. Five montmorillonites extracted from bentonites of different provenance were saturated with Li+ and heated to 300°C. Initial montmorillonites and montmorillonites with reduced layer charge (RCM) were characterized by comprehensive mineralogical analysis supplemented by CEC measurements, surface-area measurements by Ar adsorption, and 7Li, 27Al, and 29Si magic-angle spinning nuclear magnetic resonance spectroscopy (MAS NMR). The CEC of the initial montmorillonites varied between 89 and 130 cmol(+)/kg while the CEC of the RCM prepared at 300°C varied between 8 and 25 cmol(+)/kg. The lateral dimension of the 2:1 layers varied between 70 and 200 nm. The greatest decrease in CEC was observed for the montmorillonite with the largest diameter of the 2:1 layers and the smallest decrease was observed for the montmorillonite with the smallest diameter of the 2:1 layers. 7Li MAS NMR revealed an axially symmetric chemical environment of the hydrated interlayer Li+ with ηΔ = 0 for the chemical shift anisotropy tensor for unheated montmorillonites with >33% tetrahedral layer charge (ξ). The chemical environment is typical of innersphere hydration complexes of interlayer Li+. An axially non-symmetric chemical environment of the interlayer Li+ with ηCS of close to one was observed for all RCM. While the remaining CEC of RCM prepared at 300°C reflected the variable CEC at the edges, and thus the lateral size or aspect ratio of the 2:1 layers, the hydration complex of interlayer Li+ was strongly determined by the isomorphic substitutions in the dioctahedral 2:1 layers.
Similar content being viewed by others
References
Alba, M.D., Alvero, R., Becerro, A.I., Castro, M.A., and Trillo, J.M. (1998) Chemical behavior of lithium ions in reexpanded Li-montmorillonite. The Journal of Physical Chemistry (B), 102, 2207–2213.
Alvero, R., Alba, M.D., Castro, M.A., and Trillo, J.M. (1994) Reversible migration of lithium in montmorillonite. The Journal of Physical Chemistry, 98, 7848–7853.
Bak, M., Ramussen, J.T., and Nielsen, N.C. (2000) SIMPSON: A general simulation program for solid-state NMR spectroscopy. Journal of Magnetic Resonance, 147, 296–330.
Becerro, A.I., Mantovani, M., and Escudero, A. (2009) Mineralogical stability of phyllosilicates in hyperalkaline fluids: Influence of layer nature, octahedral occupation and presence of tetrahedral Al. American Mineralogist, 94, 1187–1197.
Begaudeau, K., Morizet, Y., Florian, P., Paris, M., and Mercier, J.-C. (2012) Solid-state NMR analysis of Febearing minerals: Implications and applications for earth sciences. European Journal of Mineralogy, 24, 535–550.
Betega de Paiva, L., Morales, A.R., and Diaz, F.R.V. (2008) Organoclays: Properties, preparation and applications. Applied Clay Science, 42, 8–24.
Breen, C., Madejová, J., and Komadel, P. (1995) Characterisation of moderately acid-treated, size-fractionated montmorillonites using IR and MAS NMR spectroscopy and thermal analysis. Journal of Materials Chemistry, 5, 469–474.
Breen, C., Watson, R., Madejová, J., Komadel, P., and Klapyta, Z. (1997) Acid-activated organoclays: Preparation, characterization and catalytic activity of acidtreated tetra-alkyammonium exchanged smectites. Langmuir, 13, 6473–6479.
Brunauer, S., Emmett, P.H., and Teller, E. (1932) Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, 60, 309–319.
Bujdák, J., Slosiariková, H., Nováková, L., and Čičel, B. (1991) Fixation of lithium cations in montmorillonite. Chemical Papers, 45, 499–507.
Cadars, S., Guégan, R., Garaga, M.N., Bourrat, X., Le Forestier, L., Fayon, F., Huynh, T.V., Allier, T., Nour, Z., and Massiot, D. (2012) New insights into the molecular structures, compositions, and cation distributions in synthetic and natural montmorillonite clays. Chemistry of Materials, 24, 4376–4389.
Calvet, R. and Prost, R. (1971) Cation migration into empty octahedral sites and surface properties of clays. Clays and Clay Minerals, 19, 175–186.
Carroll, D. and Starkey, H.C. (1971) Reactivity of clay minerals with acids and alkalis. Clays and Clay Minerals, 19, 321–333.
Chang, F.-R.C., Skipper, N.T., and Sposito, G. (1997) Monte Carlo and molecular dynamics simulations of interfacial structure in lithium-montmorillonite hydrates. Langmuir, 13, 2074–2082.
Delavernhe, L., Steudel, A., Darbha, G.K., Schäfer, T., Schuhmann, R., Wöll, C., Geckeis, H., and Emmerich, K. (2015) Influence of mineralogical and morphological properties on the cation exchange behavior of dioctahedral smectites. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 481, 591–599.
Drits, V.A., Besson, G., and Muller, F. (1995) An improved model for structural transformations of heat-treated aluminous dioctahedral 2:1 layer silicates. Clays and Clay Minerals, 43, 718–731.
Eisenhour, D.D. and Brown, R.K. (2009) Bentonite and its impact on modern life. Elements, 5, 83–88.
Emmerich, K., Madson, F.T., and Kahr, G. (1999) Dehydroxylation behavior of heat-treated and steam-treated homoionic cis-vacant montmorillonites. Clays and Clay Minerals, 47, 591–604.
Emmerich, K. (2011) Thermal analysis in the characterization and processing of industrial minerals. Pp. 129–170 in: Advances in the Characterization of Industrial Minerals (G.E. Christidis, editor). Volume 9, EMU Notes in Mineralogy, European Mineralogical Union and the Mineralogical Society of Great Britain & Ireland, Twickenham, UK.
Emmerich, K., Plötze, M., and Kahr, G. (2001) Reversible collapse and Mg2+ release of de- and rehydroxylated homoionic cis-vacant montmorillonites. Applied Clay Science, 19, 143–154.
Emmerich, K., Wolters, F., Kahr, G., and Lagaly, G. (2009) Clay profiling: The classification of montmorillonites. Clays and Clay Minerals, 57, 104–114.
Fysh, S.A., Cashion, J.D., and Clark, P.E. (1983) Mössbauer effect studies of iron in kaolin: I Structural iron. Clays and Clay Minerals, 31, 285–292.
Gates, W.P., Komadel, P., Madejová, J., Bujdák, J., Stucki, J.W., and Kirkpatrick, R.J. (2000) Electronic and structural properties of reduced-charge montmorillonites. Applied Clay Science, 16, 257–271.
Greathouse, J. and Sposito, G. (1998) Monte Carlo and molecular dynamics studies of interlayer structure in Li(H2O)3 — smectites. Journal of Physical Chemistry B, 102, 2406–2414.
Greene-Kelly, R. (1952) A test for montmorillonite. Nature, 170, 1130–1131.
Gregg, S.J. and Sing, K.S.W. (1991) Adsorption, Surface Area and Porosity. Academic Press, London.
Harvey, C.C. and Lagaly, G. (2013) Industrial applications. Pp. 451–490 in: Handbook of Clay Science — Part B Techniques and Applications (F. Bergaya and G. Lagaly, editors) second edition. Developments in Clay Science Volume 5B, Elsevier, Oxford.
Hofmann, U. and Klemen, R. (1950) Verlust der Austauschfähigkeit von Lithium-Ionen an Bentonit durch Erhitzung. Zeitschrift für Anorganische und Allgemeine Chemie, 262, 95–99.
Hrobáriková, J. and Komadel, P. (2002) Sorption properties of reduced charge montmorillonites. Geologica Carpathica, 53, 93–98.
Hrobárikovaá, J., Madejovaá, J., and Komadel, P. (2001) Effect of heating temperature on Li-fixation, layer charge and properties of fine fractions of bentonites. Journal of Materials Chemistry, 11, 1452–1457.
Jaynes, W.F. and Bigham, J.M. (1987) Charge reduction, octahedral charge, and lithium retention in heated, Lisaturated smectites. Clays and Clay Minerals, 35, 440–448.
Jozefaciuk, G. and Bowanko, G. (2002) Effect of acid and alkali treatments on surface areas and adsorption energies of selected minerals. Clays and Clay Minerals, 50, 771–783.
Karakassides, M.A., Madejová, J., Arvaiová, B., Bourlinos, A., Petridis, D., and Komadel, P. (1999) Location of Li(I), Cu(II) and Cd(II) in heated montmorillonite: evidence from specular reflectance infrared and electron spin resonance spectroscopies. Journal of Materials Chemistry, 9, 1553–1558.
Kleeberg, R. and Bergmann, J. (2002) Quantitative phase analysis using the Rietveld method and a fundamental parameter approach. Powder Diffraction: Proceedings of the II International School on Powder Diffraction. IACS, Kolkata, India.
Komadel, P. (2003) Chemically modified smectites. Clay Minerals, 38, 127–138.
Komadel, P., Janek, M., Madejová, J., Weekes, A., and Breen, C. (1997) Acidity and catalytic activity of mildly acidtreated Mg-rich montmorillonite and hectorite. Journal of the Chemical Society, Faraday Transactions, 93, 4207–4210.
Komadel, P., Madejová, J., and Bujdák, J. (2005) Preparation and properties of reduced-charge smectites — A review. Clays and Clay Minerals, 53, 313–334.
Komarneni, S., Fyfe, C.A., Kennedy, G.J., and Strobl, H. (1986) Characterization of synthetic and naturally occurring clays by 27Al and 29Si magic-angle spinning NMR spectroscopy. Journal of the American Ceramic Society, 69, C45–47.
Köster, H.M. (1977) Die Berechnung kristallchemischer Strukturformeln von 2:1-Schichtsilikaten unter Berücksichtigung der gemessenen Zwischenschichtladungen und Kationenumtausch-kapazitäten, sowie der Darstellung der Ladungsverteilung in der Struktur mittels Dreiecks-koordinaten. Clay Minerals, 12, 45–54.
Lagaly, G. (1994) Layer charge determination by alkylammonium ions. Pp. 1–46 in: Layer Charge Characteristics of 2:1 Silicate Clay Minerals (A.R. Mermut, editor). The Clay Minerals Society, Boulder, Colorado, USA.
Lagaly, G. and Weiss, A. (1971) Anordnung und Orientierung kationischer Tenside auf ebenen Silicatoberflächen Teil IV. Kolloid-Zeitschrift und Zeitschrift für Polymere, 243, 48–55.
Lippmaa, E., Mägi, M., Samoson, A., Engelhardt, G., and Grimmer, A.-R. (1980) Structural studies of silicates by solid-state high-resolution 29Si NMR. Journal of the American Chemical Society, 102, 4889–4893.
Luca, V., Cardile, C.M., and Meinhold, R.H. (1989) High-resolution multinuclear NMR study of cation migration in montmorillonite. Clay Minerals, 24, 115–119.
Madejová, J., Bujdák, J., Gates, W.P., and Komadel, P. (1996) Preparation and infrared spectroscopic characterization of reduced charge montmorillonite with various Li contents. Clay Minerals, 31, 233–241.
Madejová, J., Arvaiová, B., and Komadel, P. (1999) FTIR spectroscopic characterization of thermally treated Cu2+, Cd2+, and Li+ montmorillonites. Spectrochimica Acta Part A, 55, 2467–2476.
Madejová, J., Bujdák, J., Petit, S., and Komadel, P. (2000) Effects of chemical composition and temperature of heating on the infrared spectra of Li-saturated dioctahedral smectites. (I) Mid-infrared region. Clay Minerals, 35, 739–751.
Mägi, M., Lippmaa, E., Samoson, A., Engelhardt, G., and Grimmer, A.-R. (1984) Solid-state high-resolution silicon-29 chemical shifts in silicates. Journal of Physical Chemistry, 88, 1518–1522.
Massiot, D., Fayon, F., Capron, M., King, I., Le Calvé, S., Alonso, B., Durand, J.-O., Bujoli, B., Gan, Z., and Hoatson, G. (2002) Modelling one- and two-dimensional solid state NMR spectra. Magnetic Resonance in Chemistry, 40, 70–76.
Mehra, O.P. and Jackson, M.L. (1960) Iron oxide removal from soils and clays by dithionite-citrate-system buffered with sodium bicarbonate. 7thNational Conference on Clays and Clay Minerals, 317–327.
Meier, L.P. and Kahr, G. (1999) Determination of the cation exchange capacity (CEC) of clay minerals using the complexes of copper(II) ion with triethylenetetramine and tetraethylenepentamine. Clays and Clay Minerals, 47, 386–388.
Mignon, P., Ugliengo, P., Sodupe, M., and Hernandez, E.R. (2010) Ab initio molecular dynamics study of the hydration of Li+, Na+, and K+ in a montmorillonite model. Influence of isomorphic substitution. Physical Chemistry Chemical Physics, 12, 688–697.
Mosser, C., Michot, L.J., Villieras, F., and Romeo, M. (1997) Migration of cations in copper(II)-exchanged montmorillonite and laponite upon heating. Clays and Clay Minerals, 45, 789–802.
Murad, E. (1998) Clays and clay minerals: What can Mössbauer spectroscopy do to help understand them? Hyperfine Interactions, 117, 39–70.
Murad, E. and Johnston, J.H. (1987) Iron oxides and oxyhydroxides. Pp. 507–582 in: Mössbauer Spectroscopy Applied to Inorganic Chemistry (G.J. Long, editor). Vol. 2, Plenum, New York.
Murad, E. and Schwertmann, U. (1986) Influence of Al substitution and crystal size on the room-temperature Mössbauer spectrum of hematite. Clays and Clay Minerals, 34, 1–6.
Novák, I. and Číčel, B. (1978) Dissolution of smectites in hydrochloric acid: II. Dissolution rate as a function of crystallochemical composition. Clays and Clay Minerals, 26, 341–344.
Oldfield, E., Kinsey, R.A., Smith, K.A., Nichols, J.A., and Kirkpatrick, R.J. (1983) High-resolution NMR of inorganic solids — influence of magnetic centers on magic-angle sample-spinning lineshapes in some natural aluminosilicates. Journal of Magnetic Resonance, 51, 325–329.
Olis, A.C., Malla, P.B., and Douglas, L.A. (1990) The rapid estimation of the layer charges of 2:1 expanding clays from a single alkylammonium ion expansion. Clay Minerals, 25, 39–50.
Petrick, K. (2011) How does mineralogy control the technical properties of paper kaolins and ceramic clays? PhD thesis, Fakultät für Bauingenieur-, Geo- und Umweltwissenschaften, Universität Karlsruhe, Germany.
Sanz, J. and Robert, J.-L. (1992) Influence of structural factors on 29Si and 27Al NMR chemical shifts of phyllosilicates 2:1. Physics and Chemistry of Minerals, 19, 39–45.
Sanz, J. and Serratosa, J.M. (1984) 29Si and 27Al high-resolution MAS-NMR spectra of phyllosilicates. Journal of the American Chemical Society, 106, 4790–4793.
Schultz, L.G. (1969) Lithium and potassium absorption, dehydroxylation temperature, and structural water content of aluminous smectites. Clays and Clay Minerals, 17, 115–149.
Skipper, N.T., Sposito, G., and Chang, F.-R.C. (1995) Monte Carlo simulation of interlayer molecular structure in swelling clay minerals. 2. Monolayer hydrates. Clays and Clay Minerals, 43, 294–303.
Skoubris, E.N., Chryssikos, G.D., Christidis, G.E., and Gionis, V. (2013) Structural characterization of reduced-charge montmorillonites — evidence based on FTIR spectroscopy, thermal behavior, and layer-charge systematics. Clays and Clay Minerals, 61, 83–97.
Sposito, G., Prost, R., and Gaultier, J.P. (1983) Infrared spectroscopic study of adsorbed water on reduced-charge montmorillonites. Clays and Clay Minerals, 31, 9–16.
Steudel, A. (2009) Selection strategy and modification of layer silicates for technical applications. PhD thesis, Karlsruher Mineralogische und Geochemische Hefte (36), Schriftenreihe des Instituts fü r Mineralogie und Geochemie, Universitä t Karlsruhe (TH), Germany.
Steudel, A., Batenburg, L.F., Fischer, H.R., Weidler, P.G., and Emmerich, K. (2009) Alteration of swelling clay minerals by acid activation. Applied Clay Science, 44, 105–115.
Steudel, A. and Emmerich, K. (2013) Strategies for the successful preparation of homoionic smectites. Applied Clay Science, 75-76, 13–21.
Theng, B.K.G., Hayashi, S., Soma, M., and Seyama, H. (1997) Nuclear magnetic resonance and X-ray photoelectron spectroscopic investigation of lithium migration in montmorillonite. Clay and Clay Minerals, 45, 718–723.
Tkáč, I., Komadel, P., and Müller, D. (1994) Acid-treated montmorillonites — A study by 29Si and 27Al MAS NMR. Clay Minerals, 29, 11–19.
Tournassat, C., Neaman, A., Villiéras, F., Bosbach, D., and Charlet, L. (2003) Nanomorphology of montmorillonite particles: Estimation of the clay edge sorption site density by low-pressure gas adsorption and AFM observations. American Mineralogist, 88, 1989–1995.
Tributh, H. and Lagaly, G. (1986a) Aufbereitung und Identifizierung von Boden und Lagerstättentonen Teil I — Aufbereitung der Proben im Labor. GIT Fachzeitschrift für das Laboratorium, 30, 524–529.
Tributh, H. and Lagaly, G. (1986b) Aufbereitung und Identifizierung von Boden und Lagerstättentonen Teil II — Korngroßenanalyse und Gewinnung von Tonsubfraktionen. GIT Fachzeitschrift für das Laboratorium, 30, 771–776.
Trillo, J.M., Alba, M.D., Alvero, R., and Castro, M.A. (1993) Reexpansion of collapsed Li-montmorillonite; Evidence on the location of Li+ ions. Journal of the Chemical Society, Chemical Communications, 24, 1809–1811.
Wagner, F.E. and Kyek, A. (2004) Mössbauer spectroscopy in archeology: Introduction and experimental considerations. Hyperfine Interactions, 154, 5–33.
Wang, J., Zeng, F.G., and Wang, J.X. (2006) Molecular dynamics simulation studies of interlayered structure in lithium-, sodium- and potassium-montmorillonite hydrate. Acta Chimica Sinica, 64, 1654–1658.
Weiss, C.A. (Jr.), Altaner, S.P., and Kirkpatrick, R.J. (1987) High-resolution 29Si NMR spectroscopy of 2:1 layer silicates: Correlations among chemical shift, structural distortions, and chemical variations. American Mineralogist, 72, 935–942.
White, G.N. and Zelazny, L.W. (1988) Analysis and implications of the edge structure of dioctahedral phyllosilicates. Clays and Clay Minerals, 36, 141–146.
Whitney, D.L. and Evans, B.W. (2010) Abbreviations for names of rock-forming minerals. American Mineralogist, 95, 185–187.
Wolters, F. (2005) Classification of montmorillonites. PhD. thesis, Fakultät für Bauingenieur-, Geo - und Umweltwissenschaften, Universität Karlsruhe, Germany.
Wolters, F. and Emmerich, K. (2007) Thermal reactions of smectites — relation of dehydroxylation temperature to octahedral structure. Thermochimica Acta, 462, 80–88.
Wolters, F., Lagaly, G., Kahr, G., Nüesch, R., and Emmerich, K. (2009) A comprehensive characterization of dioctahedral smectites. Clays and Clay Minerals, 57, 115–133.
Xi, Y., Ding, Z., He, H., and Frost, R.L. (2004) Structure of organoclays — an X-ray diffraction and thermogravimetric analysis study. Journal of Colloid and Interface Science, 277, 116–120.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steudel, A., Heinzmann, R., Indris, S. et al. CEC and 7Li MAS NMR Study of Interlayer Li+ in the Montmorillonite—Beidellite Series at Room Temperature and After Heating. Clays Clay Miner. 63, 337–350 (2015). https://doi.org/10.1346/CCMN.2015.0630501
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2015.0630501