Abstract
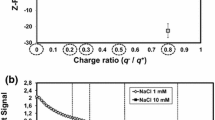
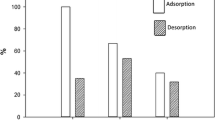
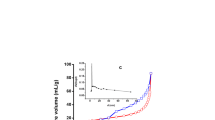
Due to the increased importance of bionanocomposites, protamine and papain proteins were adsorbed on Na+- and on Cs+-exchanged saponite from aqueous solution. Protein analysis of equilibrium solutions and thermogravimetric analyses of biocomposites were used to prepare adsorption isotherms. Based on the isotherm shape, and on the amounts of protein adsorbed and the amounts of Na+ and Cs+ released, the initial protein sorption apparently was due to ion exchange. Additional sorbed protein was weakly retained and could be removed by washing with water. From ion exchange, the average charge of the protamine adsorbed was estimated to be +13.1 to +13.5. Similar papain measurements could not be made due to partial decomposition. Quantitatively, protamine was adsorbed at levels up to 400 mg/g on Na+-saponite and 200 mg/g on Cs+-saponite. The maximum protamine adsorption was 650 to 700 mg/g for Na+-saponite and 350–400 mg/g for Cs+-saponite. Protamine was sorbed to edge surfaces and the basal spacing of the interlamellar region of saponite was 1.75 nm. Protamine displaced only 36% of the Cs+ in Cs+-saponite and expanded the interlamellar region by 36% for a basal spacing of 1.6 nm. Papain sorption to Na+-saponite occurred by a two-step process: (1) adsorption to saponite particle external surfaces followed, (2) by partial intercalation. Quantitatively, Papain was adsorbed up to 100 mg/g for Na+-and Cs+-saponite. Greater initial papain concentrations resulted in a 450 mg/g maximum for Na+-saponite, but no increase above 100 mg/g for Cs+-saponite. Papain apparently only sorbed to external Cs+-saponite surfaces that were estimated to be 33–40 m2/g. Step-wise thermal decomposition of the saponite-protein composites occurred between 300 and 800°C.
Similar content being viewed by others
References
Amemiya, S., Yang, X., and Wazenegger, T.L. (2003) Voltammetry of phase transfer of polypeptide protamines across polarized liquid/liquid interfaces. Journal of the American Chemical Society, 125, 832–833.
Blomberg, E., Claesson, P.M., Froberg, J.C., and Tilton, R.D. (1994) Interaction between adsorbed layers of lysozyme with the surface force technique. Langmuir, 10, 2325–2334.
Brack, A. (2006) Clay minerals and the origin of life. Pp. 379–391 in: Handbook of Clay Sciences (F. Bergaya, G. Lagaly, and B.K.G. Theng, editors). Elsevier, Amsterdam.
Gray, J.J. (2004) The interaction of proteins with solid surfaces. Current Opinion in Structural Biology, 14, 110–115.
Harpaz, Y., Gerstein, M., and Chothia, C. (1994) Volume changes on protein-folding. Structure, 2, 641–649.
Hudson, S., Magnier, E., Cooney, J., and Hodnett, B.K. (2005) Methodology for the Immobilization of Enzymes on Porous Materials. Journal of Physical Chemistry B, 109, 19496–19506.
Jaynes, W.F. and Boyd, S.A. (1991) Hydrophobicity of siloxane surfaces in smectites as revealed by aromatic hydrocarbon adsorption from water. Clays and Clay Minerals, 39, 428–436.
Kim, D.T., Blanch, H.W., and Radke, C.J. (2002) Direct imaging of lysozyme onto mica by AFM. Langmuir, 18, 5841–5850.
Laird, D.A., Barriuso, E., Dowdy, R.H., and Koskinen, W.C. (1992) Adsorption of atrazine on smectites. Soil Science Society of America Journal, 56, 62–67.
Maes, A. and Cremers, A. (1978) Charge density effects in ion exchange. Part 2. Homovalent exchange equilibria. Journal of the Chemical Society. Faraday Transactions, 74, 1234–1242.
Mortland, M.M., Fripiat, J.J., Chaussidon, J., and Uytterhoeven, J.B. (1963) Interaction between ammonia and the expanding lattices of montmorillonite and vermiculite. Journal of Physical Chemistry, 67, 248–258.
Nulens, K.H.L., Toufar, H., Janssens, G.O.A., Schoonheydt, R.A., and Johnston, C.T. (1996) Clay minerals and clay mineral-water interaction: a combined EEM-Monte Carlo study. Pp. 116–133 in. The latest Frontiers of Clay Chemistry. Proceedings of the Sapporo Conference on the Chemistry of Clays and Clay Minerals (Sapporo, Japan 1996), (A. Yamagishi, A. Aramata and M. Taniguchi, editors), The Smectite Forum of Japan, Sendai.
Ottensmeyer, F.P., Whiting, R.F., and Kron, A.P. (1975) Three-dimensional structure of herring sperm protamine Y-I with the aid of dark field electron microscopy. Proceedings of the National Academy of Sciences of the United States of America, 72, 4953–4955.
Quiquampoix, H., Staunton, S., Baron, M.-H., and Ratcliffe, R.G. (1993) Interpretation of the pH dependence of protein adsorption on clay surfaces. Colloids and Surfaces A, 75, 85–93.
Rasor, P. (2000) Immobilized enzymes in enantioselective organic synthesis. Pp. 97–122 in: Chiral Catalyst Immobilization and Recycling (D. De Vos, I.F.J. Vankelecom, and P.A. Jacobs, editors). Wiley-VCH, Weinheim, Germany.
Ruiz-Hitzky, E., Ariya, K., and Lvov, J.M. (2008) Bio-inorganic Hybrid Materials. Strategies, Synthesis, Characterization and Applications. Wiley, Weinheim, Germany, 500 pp.
Szabó, T., Szekeres, M., Dékány, I., Jackers, C., De Feyter, S., Johnston, C.T., and Schoonheydt, R.A. (2007) Layer-by-layer construction of ultrathin hybrid films with proteins and clay minerals. Journal of Physical Chemistry C, 111, 12730–12740.
Theng, B.K.G. (1974) Clay-Organic Interactions, A. Hilger, London, 343 pp.
Trojanek, A., Langmaier, J., Samcova, E., and Samec, Z. (2007) Counterion binding to protamine polyion at a polarized liquid-liquid interface. Journal of Electroanalytical Chemistry, 603, 235–242.
Van Olphen, H. and Fripiat, J.J. (1979) Data Handbook for Clay Materials and other Non-Metallic Materials, Pergamon, Oxford, UK, 346 pp.
Wertz, C.F. and Santore, M.M. (2001) Effect of surface hydrophobicity on adsorption and relaxation kinetics of albumin and fibrinogen: single species and competitive adsorption. Langmuir, 17, 3006–3016.
Wertz, C.F. and Santore, M.M. (2002a) Fibrinogen adsorption on hydrophilic and hydrophobic surfaces: geometrical and energetical aspects of interfacial relaxations. Langmuir, 18, 706–715.
Wertz, C.F. and Santore, M.M. (2002b) Adsorption and reorientation kinetics of lysozyme on hydrophobic surfaces. Langmuir, 18, 1190–1199.
Zhao, X.S., Bao, X.Y., Guo, W., and Lei, F.Y. (2006) Immobilizing catalysts on porous materials. Materials Today, 9, 32–39.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szabó, T., Mitea, R., Leeman, H. et al. Adsorption of protamine and papain proteins on saponite. Clays Clay Miner. 56, 494–504 (2008). https://doi.org/10.1346/CCMN.2008.0560502
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2008.0560502