Abstract
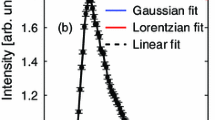
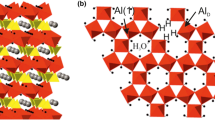
Using 19F magic angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy, we show that most of the fluoride present in the KGa-lb reference kaolinite from Washington County, Georgia, occurs as a surface-adsorbed species bonded to Al. This surface fluoride can be removed from the <2 µm fraction by acid wash, but is largely retained in the coarse fraction. Correlation of integrated 19F NMR peak intensities with fluoride sorption experiments indicates a bulk F content of ∼144 ppm for KGa-1b, of which ∼30% substitutes for hydroxyl sites in the mineral structure and the remaining 70% occurs adsorbed on particle surfaces, corresponding to an edge surface fluoride density of ∼0.7 F− nm−2. 19F{27Al} TRAPDOR (TRAnsfer of Populations in DOuble Resonance) NMR data for the original kaolinite and for products of F− sorption experiments at pH 4 show that all of the observed 19F signals arise from fluoride bonded to Al atoms. Furthermore, bridging Al-F-Al sites and terminal Al-F give distinctly different TRAPDOR fractions allowing assignment of resolved peaks based on the number of Al in the first coordination sphere. This result was confirmed for fluoride adsorbed to the surface of gibbsite from aqueous suspension. No evidence was found for Si-F-type environments on the kaolinite surfaces.
Similar content being viewed by others
References
Agarwal, M., Rai, K., Shrivastav, R., and Dass, S. (2002) A study on fluoride sorption by montmorillonite and kaolinite. Water Air and Soil Pollution, 141, 247–261.
Bar-Yosef, B., Afik, I., and Rosenberg, R. (1988) Fluoride sorption by montmorillonite and kaolinite. Soil Science, 145, 194–200.
Bickmore, B.R., Nagy, K.L., Sandlin, P.E., and Crater, T.S. (2002) Quantifying surface areas of clays by atomic force microscopy. American Mineralogist, 87, 780–783.
Bodor, A., Toth, I., Banyai, I., Szabo, Z., and Hefter, G.T. (2000) 19F NMR study of the equilibria and dynamics of the Al3+/F− system. Inorganic Chemistry, 39, 2530–2537.
Bower, C.A. and Hatcher, J.T. (1967) Adsorption of fluoride by soils and minerals. Soil Science, 103, 151–155.
Brady, P.V., Cygan, R.T., and Nagy, K.L. (1996) Molecular controls on kaolinite surface charge. Journal of Colloid and Interface Science, 183, 356–364.
Chupas, P.J., Ciraolo, M.F., Hanson, J.C., and Grey, C.P. (2001) In situ X-ray diffraction and solid-state NMR study of the fluorination of γ-Al2O3 with HCF2Cl. Journal of the American Chemical Society, 123, 1694–1702.
Chupas, P.J., Corbin, D.R., Rao, V.N.M., Hanson, J.C., and Grey, C.P. (2003) A combined solid-state NMR and diffraction study of the structures and acidity of fluorinated aluminas: Implications for catalysis. Journal of Physical Chemistry B, 107, 8327–8336.
Davis, J.A. and Kent, D.B. (1990) Surface complexation modeling in aqueous geochemistry. Pp. 177–260 in: Interface Geochemistry (M.F. Hochella and A.F. White, editors). Reviews in Mineralogy, 23, Mineralogical Society of America, Washington, D.C.
Edmunds, W.M., and Smedley, P.L. (2005) Fluoride in natural waters Pp. 301–330 in: Essentials of Medical Geology: Impacts of the Natural Environment on Public Health (O. Selinus, editor). Academic Press, New York.
Fischer, L., Harle, V., Kasztelan, S., and de la Caillerie, J.B.D. (2000) Identification of fluorine sites at the surface of fluorinated γ-alumina by two-dimensional MAS NMR. Solid State Nuclear Magnetic Resonance, 16, 85–91.
Grey, C.P. and Vega, A.J. (1995) Determination of the quadrupole coupling constant of the invisible aluminum spins in zeolite HY with 1H/27Al TRAPDOR NMR. Journal of the American Chemical Society, 117, 8232–8242.
Hiemstra, T. and van Riemsdijk, W.H. (2000) Fluoride adsorption on goethite in relation to different types of surface sites. Journal of Colloid and Interface Science, 225, 94–104.
Huertas, F.J., Chou, L., and Wollast, R. (1998) Mechanism of kaolinite dissolution at room temperature and pressure: Part I. surface speciation. Geochimica et Cosmochimica Acta, 62, 417–431.
Huve, L., Delmotte, L., Martin, P., Ledred, R., Baron, J., and Saehr, D. (1992) 19F MAS-NMR study of structural fluorine in some natural and synthetic 2:1 layer silicates. Clays and Clay Minerals, 40, 186–191.
Kau, P.M.H., Smith, D.W., and Binning, P.J. (1997) The dissolution of kaolin by acidic fluoride wastes. Soil Science, 162, 896–911.
Kau, P.M.H., Smith, D.W., and Binning, P. (1998) Experimental sorption of fluoride by kaolinite and bentonite. Geoderma, 84, 89–108.
Labouriau, A., Kim, Y.W., Chipera, S., Bish, D.L., and Earl, W.L. (1995) A 19F nuclear magnetic resonance study of natural clays. Clays and Clay Minerals, 43, 697–704.
Liu, Y. and Tossell, J. (2003) Possible Al-F bonding environment in fluorine-bearing sodium aluminosilicate glasses: From calculation of 19F NMR shifts. Journal of Physical Chemistry B, 107, 11280–11289.
Meenakshi and Maheshwari, R.C. (2006) Fluoride in drinking water and its removal. Journal of Hazardous Materials, 137, 456–463.
Nordin, J.P., Sullivan, D.J., Phillips, B.L., and Casey, W.H. (1999) Mechanisms for fluoride-promoted dissolution of bayerite [β-Al(OH)3(s)] and boehmite [γ-AlOOH]: 19F-NMR spectroscopy and aqueous surface chemistry. Geochimica et Cosmochimica Acta, 63, 3513–3524.
Perrott, K.W., Smith, B.F.L., and Inkson, R.H.E. (1976) Reaction of fluoride with soils and soil minerals. Journal of Soil Science, 27, 58–67.
Pulfer, K., Schindler, P.W., Westall, J.C., and Grauer, R. (1984) Kinetics and mechanism of dissolution of bayerite (γ-Al(OH)3) in HNO3-HF solutions at 298.2°K. Journal of Colloid and Interface Science, 101, 554–564.
Rosenqvist, J. and Casey, W.H. (2004) The flux of oxygen from the basal surface of gibbsite (α-Al(OH)3) at equilibrium. Geochimica et Cosmochimica Acta, 68, 3547–3555.
Rosenqvist, J., Persson, P., and Sjoberg, S. (2002) Protonation and charging of nanosized gibbsite (α-Al(OH)3) particles in aqueous suspension. Langmuir, 18, 4598–4604.
Sigg, L. and Stumm, W. (1981) The interaction of anions and weak acids with the hydrous goethite (α-FeOOH) surface. Colloids and Surfaces, 2, 101–117.
Sposito, G. (1984) The Surface Chemistry of Soils. Oxford University Press, New York, 234 pp.
Thomas, J., Glass, H.D., White, W.A., and Trandel, R.M. (1977) Fluoride content of clay minerals and argillaceous earth materials. Clays and Clay Minerals, 25, 278–284.
Vasudevan, D. and Stone, A.T. (1998) Adsorption of 4-nitrocatechol, 4-nitro-2-aminophenol, and 4-nitro-1,2-phenylenediamine at the metal (hydr)oxide/water interface: Effect of metal (hydr)oxide properties. Journal of Colloid and Interface Science, 202, 1–19.
Weerasooriya, R. and Wickramarathna, H.U.S. (1999) Modeling anion adsorption on kaolinite. Journal of Colloid and Interface Science, 213, 395–399.
Weerasooriya, R., Wickramarathne, H.U.S., and Dharmagunawardhane, H.A. (1998) Surface complexation modeling of fluoride adsorption onto kaolinite. Colloids and Surfaces A, 144, 267–273.
Wolff-Boenisch, D., Gislason, S.R., and Oelkers, E.H. (2004) The effect of fluoride on the dissolution rates of natural glasses at pH 4 and 25°C. Geochimica et Cosmochimica Acta, 68, 4571–4582.
Yu, P., Lee, A.P., Phillips, B.L., and Casey, W.H. (2003) Potentiometrie and 19F nuclear magnetic resonance spectroscopic study of fluoride substitution in the GaAl12 polyoxocation: Implications for aluminum (hydr)oxide mineral surfaces. Geochimica et Cosmochimica Acta, 67, 1065–1080.
Zeng, Q. and Stebbins, J.F. (2000) Fluoride sites in aluminosilicate glasses: High-resolution 19F NMR results. American Mineralogist, 85, 863–867.
Zutic, V. and Stumm, W. (1984) Effect of organic acids and fluoride on the dissolution kinetics of hydrous alumina: A model study using the rotating disk electrode. Geochimica et Cosmochimica Acta, 48, 1493–1503.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cochiara, S.G., Phillips, B.L. NMR spectroscopy of naturally occurring surface-adsorbed fluoride on Georgia kaolinite. Clays Clay Miner. 56, 90–99 (2008). https://doi.org/10.1346/CCMN.2008.0560108
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2008.0560108