Abstract
OH-Cr smectites were prepared with different mmol Cr/g smectite: 0.5, 1.5, 3.5, 5, 10 and 20 by treatment with hydroxy-chromium solution prepared at 60°C and one day of hydrolysis with OH/Cr = 2. The samples were characterized by X-ray diffraction (XRD), differential thermal analyses (DTA) and N2 adsorption-desorption isotherms.
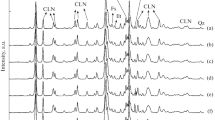
The d(001) spacings of OH-Cr-smectite were different according to Cr added/g smectite. Larger d(001) spacings: 1.95, 2.05 and 2.07 nm were obtained with 5, 10 and 20 mmol Cr per gram of sample. DTA diagrams of smectite treated with OH-Cr solution showed exothermic peak at 420°C corresponding to Cr203 (confirmed by XRD). N2 adsorption-desorption isotherms of smectite treated with different amounts of Cr preserved the same slit-shaped pores than original sample, but with different micropore volume. This behavior was maintained until treatment temperature of 380°C. The specific area of smectite was increased from 36 to 175 mVg after treatment with OH-Cr solution. The textural characteristics of OH- Cr smectite heated up to 420°C were changed. The specific area decreased and mesopore volume was produced. The different Cr added modified the structural and textural behavior.
Similar content being viewed by others
References
Barret, E. P., L. G. Joyner, and P. H. Halenda. 1961. The determination of pore volume and area distribution in porous substances. I Computation from nitrogen isotherms. J. Am. Chem. Soc. 73: 373–380.
Brindley, G. W., and S. Yamanaka. 1979. A study of hydroxychromium montmorillonites and the form of the hydroxy-chromium polymers. Am. Mineralog. 64: 830–835.
Carr, M. R. 1985. Hydration states of interlamellar chromium ions in montmorillonite. Clay & Clay Miner. 33: 357–361.
Dandy, A. J., and N. S. Nadiye-Tabbiruka. 1975. The effect of heating in vacuo on the microporosity of sepiolite. Clays & Clay Miner. 23: 428–430.
Drljaca, A., J. R. Anderson, L. Spiccia, and T. W. Turney. 1992. Intercalation of montmorillonite with individual chromium (III) hydrolytic oligomers. Inorg. Chem. 31:4894–4897.
Figueras, F. 1988. Pillared clays as catalysts. Catal. Rev. Sci. Eng. 30: 457–499.
Gregg, S. J., and K. S. W. Sing. 1991. Adsorption Surface Area and Porosity. 2nd Edition. London: Academic Press, 303 pp.
Grim, R. E., and B. Kulbicki. 1961. Montmorillonite: High temperature reactions and classification. Am. Mineralog. 46: 1329–1369.
Lippens, B. C., and J. H. de Boer. 1965. Studies on pore systems in catalysts. J. Catal. 4: 319–323.
Loeppert, R. H., and M. M. Mortland. 1979. The influence of heat-stable intercalate on the rate of dehydroxylation of smectite. Clays & Clay Miner. 27: 373–376.
Orr, C., and J. M. Dalla Valle. 1959. Fine Particle Measurement Size, Surface and Pore Volume. New York: The MacMillan Co., 27 pp.
Pierce, C. 1953. Computation of pore size from physical adsorption data. J. Phys. Chem. 57: 149–152.
Pinnavaia, T. J., M. S. Tzou, and S. D. Landau. 1985. New Chromia pillared clay catalysts. J. Am. Chem. Soc. 107: 4783–4785.
Rengasamy, P., and J. M. Oades. 1978. Intercalation of monomeric and polymeric species of metal ions with clay surfaces. III. Aluminium (III) and chromium (III). Aust. J. Soil Res. 16: 53–66.
Spiccia, L., H. Stoeckli-Evans, W. Marty, and R. Giovanoli. 1983. A new “active” chromium(III) hydroxyde: Cr2(μ- OH)2(OH)4.2H2O). Characterization and use in the preparation of salts of the (H2O)4Cr((μ-OH)2(OH)J4 ion. Crystal structure of [(H2O)4Cr(O-OH2)4] [(H3C)3C6H2SO3]4.4H2O. Inorg. Chem. 26: 474–482.
Spiccia, L., W. Marty, and R. Giovanoli. 1988. Hydrolytic trimer of chromium (III). Synthesis through chromite cleavage and use in the preparation of the “active” trimer hydroxide. Inorg. Chem. 27: 2660–2666.
Stiinzi, H., and W. Marty. 1983. Early stages of the hydrolysis of chromium (III) in aqueous solution. 1. Characterization of a tetrameric species. Inorg. Chem. 22: 2145–2150.
Stiinzi, H., L. Spiccia, F. P. Rotzinger, and W. Marty. 1989. Early stages of the hydrolysis of chromium (III) in aqueous solution. 4. Stability constant of the hydrolytic dimer, trimer and tetramer at 25°C and I = 1.0M. Inorg. Chem. 28: 66–71.
Tzou, M. S., and T. J. Pinnavaia. 1988. Chromia pillared clays. Cat. Today 2: 243–259.
Vaughan, D. E. W., and R. J. Lussier. 1980. Preparation of Molecular Sieves on Pillared Interlayered Clays (PILC). Proc. 5th. Int. Zeol. Conf. L. V. C. Rees, ed. London: Heyden Press, 94–101.
Vaughan, D. E. W. 1988. Recent development in pillared interlayered clays. Perspectives in molecular sieve science. Chapter 19. W. H. Flank and T. E. Whyte, eds. Washington, DC: American Chemical Society, 308–323.
Volzone, C., A. M. Cesio, R. M. Torres Sanchez, and E. Pereira. 1993. Hydroxy-chromium smectite. Clays & Clay Miner. 41: 702–706.
Wheler, A. 1955. Reaction Rates and Selectivity in Catalysts Pores in Catalysts, Vol. II. P. H. Emmet, ed. New York: Rainhold Publishing Corp, 118 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Volzone, C. Hydroxy-Chromium Smectite: Influence of Cr Added. Clays Clay Miner. 43, 377–382 (1995). https://doi.org/10.1346/CCMN.1995.0430312
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1995.0430312