Abstract
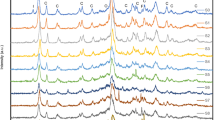
Clinoptilolite (CLN) from Bigadiç and that of calcined forms after NH4NO3 modification were investigated to demonstrate their possible usability in industrial applications. \({\text{NH}}_{4}^{ + }\)-modified forms were prepared using 0.2, 1.0, and 2.0 M NH4NO3 solutions at 90°C for 3 and 6 h, and then all samples were calcined at 400°C for 13 h. All samples were characterized using XRD, XRF, SEM and nitrogen adsorption methods. BET specific surface areas (17.8–272.9 m2 g–1) and total pore volumes (0.055–0.197 cm3 g–1) of the clinoptilolites were calculated from nitrogen adsorption isotherms obtained at 77 K by 3Flex (Micromeritics) equipment volumetrically. After increasing the molarity and processing time of the ammonium nitrate solutions, the calcination of the samples significantly affected both morphological and textural features. Compared to starting material, XRD peaks of the samples calcined at 400°C broadened and agglomeration of clinoptilolite crystals decreased. In addition, calcined clinoptilolite samples showed different ammonia adsorption capacities (4.853–5.388 mmol g–1).





Similar content being viewed by others
REFERENCES
G. Gottardi and E. Galli, Natural Zeolites (Springer, Berlin, 1985).
T. Armbruster and M. E. Gunter, in Natural Zeolites: Occurrences, Properties, Applications, Ed. by D. L. Bish and D. W. Ming, Vol. 45 of Reviews in Mineralogy and Geochemistry (Mineralog. Soc. Am., Washington, 2001) p. 1.
E. Passaglia and R. A. Sheppard, Rev. Mineral. Geochem. 45, 69 (2001). https://doi.org/10.2138/rmg.2001.45.2
F. A. Mumpton, Am. Min. 45, 351 (1960).
M. W. Ackley, R. F. Giese, and R. T. Yang, Zeolites 12, 780 (1992). https://doi.org/10.1016/0144-2449(92)90050-Y
A. Jayaraman, R. T. Yang, D. Chinn, and C. L. Munson, Ind. Eng. Chem. Res. 44, 5184 (2005). https://doi.org/10.1021/ie0492855
D. A. Kennedy and F. H. Tezel, Micropor. Mesopor. Mater. 262, 235 (2018). https://doi.org/10.1016/j.micromeso.2017.11.054
D. A. Kennedy, M. Khanafer, and F. H. Tezel, Micropor. Mesopor. Mater. 281, 123 (2019). https://doi.org/10.1016/j.micromeso.2019.03.007
Y. Garcia-Basabe, A. R. Ruiz-Salvador, G. Maurin, L. C. de Menorval, I. Rodriguez-Iznaga, and A. Gomez, Micropor. Mesopor. Mater. 155, 233 (2012). https://doi.org/10.1016/j.micromeso.2012.01.018
F. Rouquerol, J. Rouquerol and K. Sing, Adsorption by Powders and Porous Solids: Principles, Methodology, and Applications (Academic, London, 1998).
K. T. Lee, S. Bhatia, and A. R. Mohamed, Energy Sources, Part A 28, 1241 (2006). https://doi.org/10.1080/009083190933771
G. Kocasoy and G. V. Sahin, J. Environ. Sci. Health A 42, 2139 (2007). https://doi.org/10.1080/10934520701629617
A. E. Osmanlıoglu, J. Hazar. Mater. 137, 332 (2006). https://doi.org/10.1016/j.jhazmat.2006.02.013
L. L. Ames, Am. Mineral. 45, 689 (1960).
H. Kobayashi, A. Hayakawa, K. K. A. Somarathne, and E. C. Okafor, Proc. Combust. Inst. 37, 109 (2019). https://doi.org/10.1016/j.proci.2018.09.029
Ullmann’s Encyclopedia of Industrial Chemistry, Ed. by C. Ley (Wiley-VCH, Weinheim, 2000), p. 1. https://doi.org/10.1002/14356007.a02_143.pub3
A. Ghahri, F. Golbabaei, L. Vafajoo, S. M. Mireskandari, M. Yaseri, and S. J. Shahtaheri, Int. J. Environ. Res. 11, 327 (2017). https://doi.org/10.1007/s41742-017-0030-6
D. S. Karousos, A. A. Sapalidis, E. P. Kouvelos, G. E. Romanos, and N. K. Kanellopoulos, Sep. Sci. Technol. 51, 83 (2016). https://doi.org/10.1080/01496395.2015.1085880
G. E. Christidis, D. Moraetis, E. Keheyan, L. Akhalbedashvili, N. Kekelidze, R. Gevorkyan, H. Yeritsyan, and H. Sargsyan, Appl. Clay Sci. 24, 79 (2003). https://doi.org/10.1016/S0169-1317(03)00150-9
A. Ates and C. Hardacre, J. Colloid Interface Sci. 372, 130 (2012). https://doi.org/10.1016/j.jcis.2012.01.017
Y. Garcia-Basabe, I. Rodriguez-Iznaga, I. L. C. de Me-norval, P. Llewellyn, G. Maurin, D. W. Lewis, R. Binions, M. Autie, and A. R. Ruiz-Salvador, Micropor. Mesopor. Mater. 135, 187 (2010). https://doi.org/10.1016/j.micromeso.2010.07.008
T. Elysabeth and G. Ramayanti, Asian J. Chem. 31, 873 (2019). https://doi.org/10.14233/ajchem.2019.21810
D. T. Hieu, H. Kosslick, M. Riaz, A. Schulz, A. Springer, M. Frank, C. Jager, N. T. Minh Thu, and L. T. Son, Catalysts 12, 253 (2022). https://doi.org/10.3390/catal12030253
J. Liao, Y. Zhang, L. Fan, L. Chang, and W. Bao, Ind. Eng. Chem. Res. 58, 4572 (2019). https://doi.org/10.1021/acs.iecr.8b05046
M. Riaz, Ph.D. Thesis (Univ. Rostock, Germany, 2018). https://doi.org/10.18453/rosdok_id00002519
H. Kurama, A. Zimmer, and W. Reschetilowski, Chem. Eng. Technol. 25, 301 (2002). https://doi.org/10.1002/1521-4125(200203)25:3<301: :AID-CEAT301>3.0.CO;2-%23
B. Erdoğan, M. Sakızcı, and E. Yörükoğulları, Appl. Surf. Sci. 254, 2450 (2008). https://doi.org/10.1016/j.apsusc.2007.09.058
D. M. Moore and R. C. Reynolds, Jr., X-ray Diffraction and the Identification and Analysis of Clay Minerals (Oxford Univ. Press, New York, 1997).
A. Arcoya, J. A. Gonzalez, N. Travieso, and X. L. Seoane, Clay Miner. 29, 123 (1994). https://doi.org/10.1180/claymin.1994.029.1.14
S. Lowell, J. E. Shields, M. A. Thomas, and M. Thommes, Characterization of Porous Solids and Powders: Surface Area, Pore Size, and Density (Springer, Netherlands, 2006).
Y. Akdeniz and S. Ülkü, J. Therm. Anal. Calorim. 94, 703 (2008). https://doi.org/10.1007/s10973-008-9358-7
A. Brundu and G. Cerri, Micropor. Mesopor. Mater. 208, 44 (2015). https://doi.org/10.1016/j.micromeso.2015.01.029
K. Elaiopoulos, T. Perraki, and E. Grigoropoulou, Micropor. Mesopor. Mater. 134, 29 (2010). https://doi.org/10.1016/j.micromeso.2010.05.004
E. P. Favvas, C. G. Tsanaktsidis, A. A. Sapalidis, G. T. Tzilantonis, S. K. Papageorgiou, and A. C. Mitropoulos, Micropor. Mesopor. Mater. 225, 385 (2016). https://doi.org/10.1016/j.micromeso.2016.01.021
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ergürhan, O., Erdoğan, B. Effect of Calcination after NH4NO3 Modification of Natural Clinoptilolite on the Structural and Textural Properties. Russ. J. Phys. Chem. 97, 3136–3142 (2023). https://doi.org/10.1134/S0036024423130083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423130083