Abstract
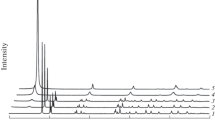
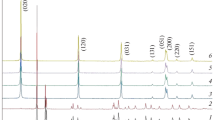
Gibbsite samples of various particle sizes (0.2–80 μm) were heated at 250°C in a series of straight-chain primary alcohols under the autogenous vapor pressure of the alcohol (alcohothermal treatment of gibbsite). The treatment in ethanol yielded pure boehmite, the morphology of which was similar to that of the boehmite obtained by hydrothermal treatment of gibbsite. In middle-range alcohols, the boehmite yields were low (50% if 80 μm gibbsite was used), and the products were contaminated by a poorly crystallized phase, having a χ-alumina-like structure. The products preserved the morphology of the originating gibbsite, this feature being similar to the thermal dehydration of gibbsite. Complete conversion to boehmite was also attained in mineral oil (a hydrocarbon mixture, which was used as a limit of higher alcohol. The morphology of the boehmite formed in this medium was identical to that of the product prepared by thermal dehydration of gibbsite in a sealed bomb without a medium. If fine particle-size gibbsite was used, the yield of boehmite decreased and the yield of the poorly crystallized phase increased in all the media.
The reaction mechanisms may be discussed in terms of the reported mechanisms for the thermal and hydrothermal formations of boehmite from gibbsite. Thus, in lower alcohols boehmite formed by a dissolution-recrystallization mechanism, whereas in middle-range or higher alcohols it formed by intra-particle hydrothermal reaction mechanism proposed by de Boer and coworkers for the thermal dehydration of gibbsite. The difference in behavior in middle-range and higher alcohols can be explained in terms of the solubility of water in the medium: In the middle-range alcohols, water molecules formed by partial dehydration of gibbsite were removed from the gibbsite particles into the medium so that dehydration proceeded in a manner similiar to that of thermal dehydration, whereas in the higher alcohols, the low solubility of water in the medium allowed the water molecules to remain on the surface of the particles, thereby promoting the complete hydrothermal formation of boehmite.
Similar content being viewed by others
References
Bauermeister, B. and Fulda, W. (1943) The Bayer process (for purification of bauxite): Aluminium 25, 97–100.
Bibby, D. M. and Dale, M. P. (1985) Synthesis of silica-sodalite from non-aqueous systems: Nature 317, 157–158.
Brindley, G. W. and Choe, J. O. (1961) The reaction series, gibbsite → chi alumina → kappa alumina → corundum: Amer. Mineral. 46, 771–785.
Brown, J. F., Clark, D., and Elliott, W. W. (1953) Thermal decomposition of alumina trihydrate, gibbsite: J. Chem. Soc, 84–88.
Bugosh, J. (1960) Esterifying the surface of alumina monohydrate plates: U.S. Patent 2, 944, 914, 10 pp.
Day, M. K. B. and Hill, V. J. (1953) The thermal transformations of the aluminas and their hydrates: J. Phys. Chem. 57, 946–950.
de Boer, J. H., Fortuin, J. M. H., and Steggerda, J. J. (1954a) The dehydration of alumina hydrates: Koninkl. Ned. Akad. Wetenschap. Proc. 57B, 170–180.
de Boer, J. H., Fortuin, J. M. H., and Steggerda, J. J. (1954b) The dehydration of alumina hydrates. II: Koninkl. Ned. Akad. Wetenschap. Proc. 57B, 435–43.
de Boer, J. H. and Linsen, B. G. (1964) Studies on pore systems in catalysts II. The shapes of pores in aluminum oxide systems: J. Catal. 3, 38–43.
de Boer, J. H., van den Heuvel, A., and Linsen, B. G. (1964) Studies on pore systems in catalysts. IV. The two causes of reversible hysteresis: Catal. 3, 268–273.
Fanelli, A. J. and Burlew, J. V. (1986) Preparation of fine alumina powder in alcohol: J. Amer. Ceram. Soc. 69, C174–C175.
Funaki, K. and Shimizu, Y. (1959) Thermal transformation of hydrothermally treated alumina hydrates: Kogyo Kagaku Zasshi 62, 782–787 (in Japanese).
Ginsberg, H., Huettig, W., and Strunk-Lichtenberg, G. (1957a) The influence of the starting material on the crystalline forms arising in the thermal decomposition and conversion of aluminum hydroxides. I. The crystalline forms of the thermal decomposition of •y-aluminum hydroxide: Z. An-org. Allgem. Chem. 293, 33–46.
Ginsberg, H., Huettig, W., and Strunk-Lichtenberg, G. (1957b) The influence of the starting material on the crystalline forms arising in the thermal decomposition and conversion of aluminum hydroxides. II. Influence of the starting material on the course of the decomposition of the 7-hydrox-ides: Z. Anorg. Allgem. Chem. 293, 204–213.
Ginsberg, H. and Koester, M. (1952) Note on the aluminum oxide monohydrate: Z. Anorg. Allgem. Chem. 271, 41–48.
Huettig, G. F. and von Wittgenstein, E. (1928) The system, alumina-water: Z. Anorg. Allgem. Chem. 171, 323–343.
Inoue, M., Kondo, Y., and Inui, T. (1986) The reaction of crystalline aluminum hydroxide in ethylene glycol: Chem. Lett, 1421–1424.
Inoue, M., Kondo, Y., and Inui, T. (1988) An ethylene glycol derivative of boehmite: Inorg. Chem. 27, 215–221.
Inui, T., Miyaké, T., Fukuda, K., and Takegami, Y. (1983) Control of pore structures of 7-alumina by the calcination of boehmite prepared from gibbsite under specific conditions: Appl. Catal. 6, 165–173.
Inui, T., Miyaké, T., and Takegami, Y. (1982) Influence of the pore structure of alumina support on the dispersed nickel particles size and C02-methanation activity: J. Jpn. Petrol. Inst. 25, 242–247.
Kubo, T. and Uchida, K. (1970) Reaction between aluminum hydroxide and methanol: Kogyo Kagaku Zasshi 73, 70–75 (in Japanese).
Lodding, W. (1969) The gibbsite dehydroxylation fork: in Thermal Analysis, R. F. Schwenker, Jr. and P. D. Gran, eds., Academic Press, New York, 1239–1250.
Naumann, R., Koehnke, K., Paulik, J., and Paulik, F. (1983) Kinetics and mechanism of the dehydration of hydrargil-lites. Part II. Thermochim. Acta 64, 15–26.
Papée, D. and Tertian, A. (1955) Thermal decomposition of hydrargillite and the constitution of the activated alumina: Bull. Soc. Chim. Fr., 983–991.
Paulik, F., Paulik, J., Naumann, R., Koehnke, K., and Pet-zold, D. (1983) Mechanism and kinetics of the dehydration of hydrargillites. Part I: Thermochim. Acta 64, 1–14.
Pokol, G., Varhegyi, G., and Verady, L. (1984) Studies on the kinetics of the gibbsite -► x-alumina reaction: Thermochim. Acta 76, 237–247.
Rouquerol, J., Rouquerol, F., and Ganteaume, M. (1975) Thermal decomposition of gibbsite under low pressures I. Formation of the boehmite phase:. J. Catal. 36, 99–110.
Rouquerol, J., Rouquerol, F., and Ganteaume, M. (1979) Thermal decomposition of gibbsite under low pressures. II. Formation of microporous alumina:. J. Catal. 57, 222–230.
Russell, A. S., Edwards, J. D., and Taylor, C. S. (1955) Solubility and density of hydrated aluminas in NaOH solutions: J. Metal. 7, 1123–1128.
Sato, T. (1960) Hydrothermal reaction of aluminum trihydrate:. J. Appl. Chem. 10, 414–417.
Shimizu, Y., Miyashige, T., and Funaki, K. (1964) Aging of aluminogel: Kogyo Kagaku Zasshi 67, 788–797 (in Japanese).
Stumpf, H. C., Russell, A. S., Newsome, J. W., and Tucker, C. M. (1950) Thermal transformations of aluminas and alumina hydrates: Ind. Eng. Chem. 42, 1398–1403.
Suzuki, M., Ito, S., and Kuwahara, T. (1981) Alcohol treatment of alumina hydrate: Shikizai 54, 742–752 (in Japanese).
Tertian, R. and Papée, D. (1958) Thermal and hydrothermal transformations of alumina: J. Chim. Phys. 55, 341–353.
Tettenhorst, R. and Hofmann, D. A. (1980) Crystal chemistry of boehmite: Clays & Clay Minerals 28, 373–380.
Thibon, H., Charrier, J., and Tertian, R. (1951) Thermal decomposition of alumina hydrates: Bull. Soc. Chim. Fr., 384–392.
Torkar, K., Worel, H., and Krischner, H. (1960) Aluminum hydroxides and oxides. III. Preparation of high-purity boehmite and bayerite in autoclaves: Monatsh. Chem. 91, 653–657.
Violante, A. and Huang, P. M. (1985) Influence of inorganic and organic ligands on the formation of aluminum hydroxides and oxyhydroxides: Clays & Clay Minerals 33, 181–192.
Wefers, K. and Bell, G. M. (1972) Oxides and hydroxides of aluminum: Alcoa Tech. Pap. 19, 36–45.
Yamaguchi, G. and Chiù, W.-C. (1968) The hydration and crystallization of p-alumina and alumina-gel in aqueous solutions of various basic reagents: Bull. Chem. Soc. Jpn. 41, 348–353.
Yamaguchi, G. and Sakamoto, K. (1959a) Hydrothermal reaction of aluminumtrihydroxides: Bull. Chem. Soc. Jpn. 32, 696–699.
Yamaguchi, G. and Sakamoto, K. (1959b) Effect of dry grinding of gibbsite: Bull. Chem. Soc. Jpn. 32, 1364–1368.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Inoue, M., Kitamura, K., Tanino, H. et al. Alcohothermal Treatments of Gibbsite: Mechanisms for the Formation of Boehmite. Clays Clay Miner. 37, 71–80 (1989). https://doi.org/10.1346/CCMN.1989.0370109
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1989.0370109