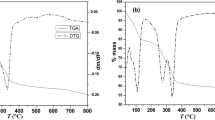
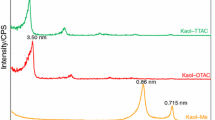
Abstract
Several new salt intercalation complexes of halloysite have been prepared and characterized using X-ray powder diffraction techniques taking into account both the position and the shape of the d001 peaks. The amount of intercalated ion in some fully complexed halloysites has been directly determined using conventional analytical techniques. The results show that less than half of the theoretical amount of salt is intercalated into the clay; the amount of salt depending on its nature and, where hydrolyzable ions are present, on the pH. Infrared spectra taken of some complexes give an indication of the nature of the interaction within interlayer space and elsewhere. The interactions are weak and are either dipolar attractions or hydrogen bonds. The ions which show the greatest tendency to intercalate with halloysite are water structure-breaking cations or hydrogen-bonding anions.
Резюме
Были получены несколько новых комплексов галлуазита, переслаивающегося с солью, которые охарактеризованы с использованием порошкового метода рентгеноструктурного анализа, причем во внимание принимались как положение, так и форма пиков d001. Количество внедрившихся ионов в некоторых полностью укомплектованных галлуазитах определялось с использованием обычных аналитических методов. Результаты показывают, что менее, чем половина теоретического количества соли внедрилась в глину. Количество соли зависит от ее природы и, если присутствуют ионы, поддающиеся гидролизу, от pH. Инфракрасные спектры некоторых комплексов указывают на природу взаимодействия в межслойном пространстве и других местах. Взаимодействия слабы и являются или дипольным притяжением, или водородными связями. Ионы, которые проявляют наибольшую тенденцию к внедрению в галлуазит, являются водными структуро-разрушающими катионами или водородо-связующими анионами.
Kurzreferat
Etliche neue Salzeinbettungskomplexe von Halloysit wurden prepariert und durch Röntgenpulverdiagramme charakterisiert, indem sowohl die Position wie auch die Form der d001 Signale in Betracht gezogen wurden. Die Menge von eingebetteten Ionen wurde unmittelbar mit konventionellen, analytischen Techniken in einigen vollkomplexierten Halloysiten bestimmt. Die Resultate zeigen, daß weniger als die Hälfte der theoretischen Menge des Salzes in den Ton eingebettet ist. Die Menge des Salzes hängt von ihrer Natur, und falls die Ionen hydrolisierbar sind, von dem pH ab. Infrarotspektra, welche von einigen Komplexen aufgenommen wurden, geben Anhaltspunkte über die Natur der Wechselwirkung in der Zwischenschicht ab. Diese Wechselwirkungen sind schwach und entweder dipolare Anziehungen oder Wasserstoffbindungen. Diejenigen Ionen, die die größte Tendenz zur Einbettung in Halloysiten zeigen, sind Wasserstruktur- brechende Kationen oder Wasserstoff-bindende Anion
Similar content being viewed by others
References
Askenasy, P. E., Dixon, T. R. and McKee, T. R. (1973) Spheroidal halloysite in a Guatemalan soil: Soil Sci. Soc. Am. Proc. 37, 799–803.
Bingham, E. C. (1941) Fluidity of electrolytes: J. Phys. Chem. 45, 885–903.
Brindley, G. W. (1961) Kaolin, serpentine, and kindred minerals. In The X-ray Identification and Crystal Structures of Clay Minerals (Edited by Brown, G.), pp. 51–131, Mineral. Soc., London.
Buswell, A. M. and Dudenbostel, B. F. (1941) Spectroscopic studies of base-exchange materials: J. Am. Chem. Soc. 63, 2554–2559.
Carr, R. M. and Chih, H. (1971) Complexes of halloysite with organic compounds: Clay Miner. 9, 153–166.
Cashen, G. H. (1959) Electric charges of kaolin: Trans. Faraday Soc. 55, 477–486.
Churchman, G. J., Aldridge, L. P. and Carr, R. M. (1972) The relationship between the hydrated and dehydrated states of an halloysite: Clays & Clay Minerals 20, 241–246.
Churchman, G. J. and Carr, R. M. (1975) The definition and nomenclature of halloysites: Clays & Clay Minerals 23, 382–388.
Dickman, S. R. and Bray, R. H. (1941) Replacement of absorbed phosphate from kaolinite by fluoride: Soil Sci. 52, 263–275.
Garrett, W. G. and Walker, G. F. (1959) The cation-exchange capacity of hydrated halloysite and the formation of halloysite-salt complexes: Clay Miner. Bull. 4, 75–80.
Hofmann, U. and Reingraber, R. (1969) Einlagerungsverbindungen in wasserarmem halloysit: Z. Anorg. Allg. Chem. 369, 208–211.
Hughes, I. R. (1966) Mineral changes of halloysite on drying: N. Z. J. Sci. 9, 103–113.
MacEwan, D. M. C. (1948) Complexes of clays with organic compounds: Trans. Faraday Soc. 44, 349–367.
McKee, T. R., Dixon, J. B., Whitehouse, G. and Harling, D. F. (1973) Study of Te Puke halloysite by a high resolution electron microscope: Proc. 31st Annu. Electron Microsc. Soc. Am. Meet., Claitor Publ., Baton Rouge, La. pp. 200–201.
Oliver, F. H. (1966) The determination of fluorine or phosphorus in organic compounds by a microtitrimetric method: Analyst 91, 771–774.
Riviere, A. (1948) On the base exchange capacity of halloysites: Summary in Clay Miner. Bull. 1, 121.
Serratosa, J. M., Hidalgo, A. and Vinas, J. M. (1963) Infrared study of the OH groups in Kaolin minerals: Proc. Int. Clay Conf., Stockholm 1, 17–26.
Verwey, E. J. W. (1942) The reciprocal effect of ions and solvent in aqueous electrolyte solutions: Ret. Trav. Chim. 61, 127–142.
Wada, K. (1958) Adsorption of alkali chloride and ammonium halide on halloysite: Soil Plant Food 4, 137–144.
Wada, K. (1959a) Oriented penetration of ionic compounds between the silicate layers of halloysite: Am. Mineral. 44, 153–165.
Wada, K. (1959b) An interlayer complex of halloysite with ammonium chloride: Am. Mineral. 44, 1237–1247.
Wada, K. (1961) Lattice expansion of kaolin minerals by treatment with potassium acetate: Am. Mineral. 46, 78–91.
Wada, K. (1965) Intercalation of water in kaolin minerals: Am. Mineral. 50, 924–941.
Weiss, A., Mehler, A., Koch, G. and Hofmann, U. (1956) Uber das anionenaustauschvermogen der tonmineralien: Z. Anorg. Allg. Chem. 284, 247–271.
Yariv, S. and Shoval, S. (1975) The nature of the interaction between water molecules and kaolin-like layers in hydrated halloysite: Clays & Clay Minerals 23, 473–474.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carr, R.M., Chaikum, N. & Patterson, N. Intercalation of Salts in Halloysite. Clays Clay Miner. 26, 144–152 (1978). https://doi.org/10.1346/CCMN.1978.0260210
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1978.0260210