Abstract
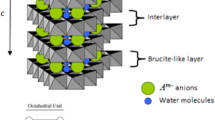
Layered double hydroxides (LDHs), also called anionic clays, consist of cationic brucite-like layers and exchangeable interlayer anions. These hydrotalcite-like compounds, with Zn and Al in the layers and chloride in the interlayer space, were prepared following the coprecipitation method at constant pH. The effect of pH, aging time and anion concentration on the intercalation of fluorophosphate \((\hbox {PO}_{3}\hbox {F}^{2-}\), FP) in the [Zn–Al] LDH was investigated. The best crystalline material, with high exchange extent, was obtained by carrying out the exchange at 25\({^{\circ }}\hbox {C}\) in a 0.03 M FP solution at pH 7 with at least 42 h of aging time. A mechanism for the FP intercalation was confirmed by X-ray diffraction, infrared spectroscopy and thermogravimetry (TG) analyses (TG and DTG curves).










Similar content being viewed by others
References
Manasse E 1915 Atti Soc. Toscana Sci. Nat. Proc. Verb. 24 92
Allmann R 1968 Acta Crystallogr. B 24 972
Taylor H F W 1969 Mineral. Mag. 37 338
Taylor H F W 1973 Mineral. Mag. 39 377
Newman A C D 1987 Chemistry of clays and clay minerals, Mineralogical Society monograph vol 6 (New York: Wiley-Interscience) p 469
Evans D G and Slade R C T 2006 Struct. Bond. 119 1
Cavani F, Trifiro F and Vaccari A 1991 Catal. Today 11 173
Rives V 2001 Layered double hydroxides: present and future (New York: Nova Science)
Braterman P S, Xu Z P and Yarberry F 2003 In S M Auerbach, K A Carrado and Dutta P K (eds) Handbook of layered materials (New York: Marcel Dekker)
Duan X and Evans D G (eds) 2006 Layered double hydroxides (Berlin: Springer)
Zümreoglu-Karan B and Ay A N 2012 Chem. Pap. 66 1
Rosenberg S P and Armstrong L 2013 D Donaldson and B E Raahauge (eds) Essential readings in light metals: alumina and bauxite vol 1 (Hoboken: John Wiley)
Fan G, Li F, Evans D G and Duan X 2014 Chem. Soc. Rev. 43 7040
Badreddine M, Legrouri A, Barroug A, De Roy A and Besse J P 1999 Mater. Lett. 38 391
Elkhattabi E, Badreddine M, Berraho M and Legrouri A 2012 Bull. Mater. Sci. 35 693
Elkhattabi E, Lakraimi M, Badreddine M, Legrouri A, Cherkaoui O and Berraho M 2013 Appl. Water Sci. 3 431
Ookubo A, Ooi K and Hayashi H 1993 Langmuir 9 1418
Ookubo A, Ooi K and Tani F 1994 Langmuir 10 407
Reichle W T 1986 Solid State Ion. 22 135
Miyata S 1980 Clay Miner. 28 50
Mering J 1949 Acta Crystallogr. 2 371
Manohara G V and Kamath P V 2014 Z. Anorg. Allg. Chem. 640 434
Bish D L and Brindley G W 1977 Am. Mineral. 62 458
Durand J, Beys L, Hillaire P, Aleonard S and Cot L 1978 Spectrochim. Acta A 34 123
Labajos F M and Rives V 1996 Inorg. Chem. 35 5313
Gnanaraj J S, Zinigrad E, Asraf L, Gottlieb H E, Sprecher M, Aurbach D and Schmidt M 2003 J. Power Sources 119 794
Mostarih R and De Roy A 2006 J. Phys. Chem. Solids 67 1058
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elkhattabi, E.H., Lakraimi, M., Berraho, M. et al. A new nanostructured material based on fluorophosphate incorporated into a zinc–aluminium layered double hydroxide by ion exchange. Bull Mater Sci 40, 745–751 (2017). https://doi.org/10.1007/s12034-017-1414-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-017-1414-0