Abstract
Background
Resection of perihilar cholangiocarcinoma (pCCA) is a complex procedure with a high risk of postoperative mortality and early disease recurrence. The objective of this study was to compare patient characteristics and overall survival (OS) between pCCA patients who underwent an R1 resection and patients with localized pCCA who received palliative systemic chemotherapy.
Methods
Patients with a diagnosis of pCCA between 1997–2021 were identified from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) registry. pCCA patients who underwent an R1 resection were compared with patients with localized pCCA (i.e., nonmetastatic) who were ineligible for surgical resection and received palliative systemic chemotherapy. The primary outcome was OS.
Results
Overall, 146 patients in the R1 resection group and 92 patients in the palliative chemotherapy group were included. The palliative chemotherapy group more often underwent biliary drainage (95% vs. 66%, p < 0.001) and had more vascular encasement on imaging (70% vs. 49%, p = 0.012) and CA 19.9 was more frequently >200 IU/L (64 vs. 45%, p = 0.046). Median OS was comparable between both groups (17.1 vs. 16 months, p = 0.06). Overall survival at 5 years after diagnosis was 20.0% with R1 resection and 2.2% with chemotherapy. Type of treatment (i.e., R1 resection or palliative chemotherapy) was not an independent predictor of OS (hazard ratio 0.76, 95% confidence interval 0.55–1.07).
Conclusions
Palliative systemic chemotherapy should be considered instead of resection in patients with a high risk of both R1 resection and postoperative mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Perihilar cholangiocarcinoma (pCCA) is a rare disease, with an annual incidence of one to two per 100,000 in Western countries.1 The median overall survival (OS) after surgical resection is approximately 30 months, and the 5-year OS approximately 30%.2,3,4 However, only approximately 15% of patients with pCCA undergo a curative intent resection.5 The majority of patients with pCCA present with metastatic disease, locally advanced disease, or are unfit to undergo major liver resection. Most experts agree that patients with metastatic (i.e., stage IV) pCCA are unlikely to benefit from a resection.6
A resection of pCCA is recommended when a complete (i.e., margin-negative [R0]) resection is likely with an acceptable 90-day postoperative mortality. Approximately one third of patients, however, undergo a histological margin-positive (i.e., R1) resection.7 The median OS after an R1 resection is approximately 18 months, and the 5-year OS is approximately 10%.8,9,10,11,12,13,14 The 90-day mortality after resection for pCCA was approximately 12% in two nationwide series but increased to 25% in patients with multiple risk factors.4,15,16 It is not known whether patients with pCCA benefit from an R1 resection compared with palliative systemic chemotherapy.
Cross-sectional imaging is inadequate to determine the biliary extent of the tumor and predict how likely an R0 resection is. Moreover, it often is uncertain on imaging whether vascular abutment requires reconstruction of the hepatic artery and portal vein to obtain a negative margin.17 A more extended resection (e.g., extended right hemihepatectomy with vascular reconstruction) is more likely to result in a negative margin but also increases the risk of postoperative mortality.
The alternative to surgical resection of localized pCCA is palliative systemic chemotherapy or best supportive care. The median OS with palliative systemic chemotherapy (the standard of care cisplatin–gemcitabine) for advanced biliary tract cancer was 11.7 months in the ABC-02 trial, albeit including patients with metastatic disease and patients with Eastern Cooperative Oncology Group (ECOG) performance status 2.18 Overall survival beyond 3 years is rarely observed after palliative systemic chemotherapy in patients with pCCA. Best supportive care (including palliative biliary drainage) is associated with a median OS of only 5 months.19
Starting from these observations, we hypothesized that patients with pCCA who underwent an R1 resection may have a similar OS compared with patients with localized pCCA who were ineligible for surgical resection and who received palliative systemic chemotherapy. The purpose of this retrospective cohort study was to compare patient characteristics and OS between patients with localized pCCA who underwent an R1 resection versus palliative systemic chemotherapy.
Methods
ENS-CCA Registry
Patients were selected from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) registry, which is a multicenter, international, collaborative research network that aims to improve the understanding of cholangiocarcinoma (intrahepatic, perihilar, and distal) and to improve patient outcomes.20,21 The registry includes consecutive patients diagnosed with pCCA at 26 referral hospitals from 11 European countries (Austria, France, Germany, Italy, Lithuania, Netherlands, Norway, Poland, Romania, Spain, and United Kingdom). Patient data, tumor characteristics, and outcomes of (non-)surgical treatment were included in the registry.
Included Patients
Patients with a diagnosis of pCCA between 1997–2021 were retrospectively included. Two cohorts of patients were selected. The first cohort included patients who underwent a curative-intent resection for pCCA with one or more positive resection margins (R1) upon histopathologic examination. Patients with R2 resection margins (macroscopic residual disease) were excluded. The second cohort included patients with localized (nonmetastatic) pCCA who were considered ineligible for surgical resection but received palliative systemic chemotherapy. Patients considered ineligible for resection had locally advanced disease on imaging (i.e., liver remnant too small or extensive vascular reconstruction needed) and/or a poor performance status. Patients were excluded in case they underwent liver transplantation or had metastatic (M1) disease on preoperative imaging, at staging laparoscopy, or laparotomy. In accordance with the AJCC 8th edition, extraregional lymph node involvement was considered distant metastatic disease.22
Patient Workup and Management
Workup and perioperative management differed across centers because of the multicenter and retrospective study design. Selected patients were treated with (neo)adjuvant therapy. Neoadjuvant therapy consisted primarily of radiotherapy (3x3.5 Gray), and adjuvant therapy consisted of (radio)chemotherapy with either gemcitabine, cisplatin, capecitabine, 5-fluorouracil, oxaliplatin, or a combination of these agents. Palliative patients with localized pCCA were treated with systemic chemotherapy, which consisted of gemcitabine, cisplatin, capecitabine, oxaliplatin, or a combination of these systemic therapies. Diagnosis of pCCA was confirmed at the histopathological level in all patients of the resection group, whereas patients who did not undergo a resection were diagnosed by brush cytology, biopsy, or high clinical suspicion (clinical presentation, serum tumor biomarkers (i.e., CA19-9 and CEA), and radiological imaging).
Definition and Outcomes
Pathology records that described the positive (R1) resection margins were considered as incomplete resections, with likely residual cancer cells in the transection surface. Overall survival was calculated from the date of diagnosis (cytologic/histologic confirmation or radiological imaging if pathology was not available) to the date of death or last follow-up.
Statistical Analysis
Categorical variables were expressed as numbers with percentages and analyzed by using the chi-squared or Fisher’s exact test. Continuous variables were reported as median with interquartile range (IQR) and were tested by using Mann-Whitney U tests. Multiple imputations were performed by using the MICE package for R (www.r-project.net). Survival curves were generated by using the Kaplan-Meier method. Differences in survival curves were tested by using the log-rank test. A Cox regression analysis was conducted to determine factors associated with OS in a multivariable model. All variables with p ≤ 0.1 were entered into the multivariable analyses by using backward selection.
RESULTS
Patient Characteristics
A total of 741 patients with localized pCCA from 25 participating centers in ten European countries were identified in the registry. Patients were excluded if they had metastatic disease at presentation (n = 300), an R2 resection (n = 9), or received best supportive care (n = 194). A flowchart is presented in Fig. 1, and the baseline characteristics of the 238 included patients are shown in Table 1. Patients who underwent a curative-intent resection with R1 resection margins upon histopathologic examination represent the R1 resection group (n = 146). Patients with localized pCCA who were ineligible for surgical resection and who received palliative systemic chemotherapy represent the palliative chemotherapy group (n = 92). In the palliative group, pathological confirmation of pCCA was obtained in 62 patients (67.4%). Of the patients who underwent a resection, 13 patients (8.9%) were treated with neoadjuvant therapy (n = 10 with radiotherapy, n = 3 with chemotherapy), and 34 patients (24.3%) received adjuvant systemic chemotherapy. The majority of the included patients was treated in the past 10 years (74.4%).
Patients in the palliative chemotherapy group had a higher body mass index (BMI) (24.9 vs. 23.5, p = 0.018) and underwent biliary stent placement more frequently prior to treatment (95% vs. 66%, p < 0.001). No difference was found in ECOG performance status. Patients who underwent palliative systemic therapy more often had vascular encasement on imaging (70% vs. 49%, p = 0.012) and CA 19.9 was more frequently >200 IU/L (64 vs. 45%, p = 0.046).
Overall Survival
Sixty-five patients (27.4%) were alive at last follow-up. The median follow-up for patients alive at the last follow-up was 22.3 months for patients in the R1 resection group and 6.4 months for patients in the palliative chemotherapy group. Postoperative mortality at 90 days was 19.9%. Median OS was 17.1 months (95% CI 10.8–23.3) for the R1 resection group and 16.0 months (95% CI 11.4–20.6) for the palliative chemotherapy group (p = 0.06; Fig. 2). Estimated survival at 6 months from diagnosis was 78.5% after R1 resection versus 91.6% after palliative systemic chemotherapy. Overall survival for R1 resection versus palliative chemotherapy at 1 year was 64.1% versus 61.9%, at 3 years 29.7% versus 12.9%, and at 5 years 20.0% versus 2.2%.
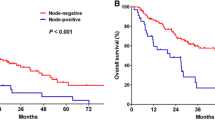
Within the resection group, a median OS of 12.5 months (95% CI 9.3–15.6) was found for patients with positive lymph nodes (N1/2) compared with 33.7 months (95% CI 25.9–41.4) median OS for negative (N0) lymph nodes (p < 0.001; Fig. 3).
Uni- and multivariable analyses are shown in Table 2. Advanced age was an independent poor prognostic factor for all patients (HR 1.02, 95% CI 1.00–1.03). Type of treatment (i.e., R1 resection or palliative chemo) was not an independent predictor of OS (HR 0.76, 95% CI 0.55–1.07).
Discussion
This study compared 146 patients who underwent an R1 resection for pCCA with 92 patients with localized pCCA who received palliative systemic chemotherapy selected from the ENS-CCA registry. Despite more vascular invasion and higher CA 19.9 levels in the palliative systemic chemotherapy group, median OS was comparable between the two groups (17.1 vs. 16.0 months, p = 0.06), and type of treatment was not an independent predictor of OS. Estimated survival at 6 months after diagnosis was lower after resection (78.5% vs. 91.6%), mainly due to postoperative mortality. Estimated survival at 5 years after diagnosis, however, was 20.0% after resection and negligible (2.2%) after palliative treatment.
The decision between resection and palliative chemotherapy for patients with localized pCCA can be challenging. Only one third of patients who undergo surgical exploration for pCCA can expect a favorable outcome, defined as an R0 resection without 90-day mortality.23 Occult metastatic or locally advanced (i.e., unresectable) disease at exploration is the most common cause of unfavorable outcome. Postoperative mortality is another important factor affecting outcome, with a 90-day mortality in nationwide studies of approximately 10%.4,15 Patients with multiple risk factors, such as advanced age, small volume of the liver remnant, and preoperative cholangitis, have a predicted 90-day postoperative mortality that may exceed 25%.24.The third cause of unfavorable outcome after resection of pCCA is an R1 resection, which is strongly associated with poor OS.
The long-term survival benefit of resection should clearly outweigh the risk of 90-day mortality. An R1 resection is a well-established poor prognostic factor.25 The median OS after an R1 resection is only approximately 18 months and the 5-year OS approximately 10%.8,9,10,11,12,13,14 In the present study, we found that the median OS for pCCA after R1 resection was similar to palliative chemotherapy. Five-year survival in the resection group, however, was clearly superior at 20.0% (vs. 2.2% in the palliative chemotherapy), at a cost of a 90-day mortality of 19.9%. This presents a difficult trade-off between long-term benefit and short-term harm for patients and their multidisciplinary team.
A prognostic model for OS after resection of pCCA found three independent poor prognostic factors: nodal disease, margin status, and moderate/poor tumor differentiation.25 These factors, however, are largely unknown when deciding between surgery and palliative systemic chemotherapy. Cure of pCCA after resection in patients with lymph node-positive disease (N+; N1 or N2) is exceedingly rare.26 Within the R1 resection group of the present study, the median OS was only 12.5 months in patients with N+ disease compared with 33.7 months in patients with N0 disease. Prognosis of N+ pCCA is so poor that in the presence of positive regional lymph nodes, resection margin status is no longer associated with OS after resection.27 Therefore, we recommend preoperative (with EUS) and intraoperative (with frozen sections) assessment of lymph node status in patients with a high risk of postoperative mortality.
One of the most ambitious goals of surgery for pCCA is to increase the chance of an R0 resection.7 Strategies, such as extended hepatectomy or routine portal vein resection, have been proposed to increase the chance of R0 resections.28 Vascular resections of the portal vein or hepatic artery may help to achieve R0 resection margins but with a substantial increase in both postoperative morbidity and mortality.29 Mizuno et al. from Japan have argued that vascular resections with reconstruction of the portal vein and/or hepatic artery should be performed in patients who often are considered as unresectable by many Western centers.30 In-hospital or 90-day mortality was slightly higher in the vascular resection group compared with the no vascular resection group (3.6% vs. 1.2%, p = 0.040). The median OS following a vascular resection was shorter (30 months) compared with no vascular resection (61 months) but still longer than the median OS of patients who did not undergo a resection (10 months). Both the postoperative mortality and long-term OS, however, were much more favorable than has been published by any Western center.
Several limitations of the present study should be acknowledged. The retrospective nature has led to selection bias involving the two study cohorts. Patients who underwent a resection differed from those who underwent palliative systemic chemotherapy; on average, the former had less advanced disease and a better performance status. This could partly explain superior 5-year OS after resection compared with palliative systemic chemotherapy. Second, the long study period may have biased results, because both surgical and palliative treatment have evolved over time. In particular, the addition of immunotherapy (durvalumab) in the TOPAZ-1 randomized controlled trial showed improved 2-year OS (24.9% vs. 10.4%) in patients with advanced biliary tract cancer.31 Third, the 90-day postoperative mortality was higher than most Western series. This could be partly explained by more extensive resections in patients with an R1 resection and the inclusion of patients from 25 centers rather than a small number of high-volume centers. Finally, not all patients in the palliative chemotherapy group had pathological confirmation of pCCA. These patients may have had nonmalignant disease, although this is unlikely given the negligible 3-year OS.
Conclusions
Patients with pCCA who underwent an R1 resection had similar median OS compared with patients with localized pCCA who were treated with palliative systemic chemotherapy. Palliative systemic chemotherapy should be considered in patients with a high risk of both R1 resection and postoperative mortality.
References
Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39(Suppl 1):19–31.
Popescu I, Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbecks Arch Surg. 2014;399(6):693–705.
Cho MS, Kim SH, Park SW, Lim JH, Choi GH, Park JS, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg. 2012;16(9):1672–9.
Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147(1):26–34.
van Keulen AM, Franssen S, van der Geest LG, de Boer MT, Coenraad M, van Driel L, et al. Nationwide treatment and outcomes of perihilar cholangiocarcinoma. Liver Int. 2021;41(8):1945–53.
Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int. 2019;39(Suppl):143–55.
van Keulen AM, Olthof PB, Cescon M, Guglielmi A, Jarnagin WR, Nadalin S, et al. Actual 10-year survival after resection of perihilar cholangiocarcinoma: what factors preclude a chance for cure? Cancers (Basel). 2021;13(24):6260.
DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–62.
Serrablo A, Tejedor L. Outcome of surgical resection in Klatskin tumors. World J Gastrointest Oncol. 2013;5(7):147–58.
Capobianco I, Rolinger J, Nadalin S. Resection for Klatskin tumors: technical complexities and results. Transl Gastroenterol Hepatol. 2018;3:69.
de Jong MC, Marques H, Clary BM, Bauer TW, Marsh JW, Ribero D, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012;118(19):4737–47.
Nakanishi Y, Tsuchikawa T, Okamura K, Nakamura T, Tamoto E, Murakami S, et al. Prognostic impact of the site of portal vein invasion in patients with surgically resected perihilar cholangiocarcinoma. Surgery. 2016;159(6):1511–9.
D’Amico FE, Mescoli C, Caregari S, Pasquale A, Billato I, Alessandris R, et al. Impact of positive radial margin on recurrence and survival in perihilar cholangiocarcinoma. Cancers (Basel). 2022;14(7):2389.
Hau HM, Meyer F, Jahn N, Rademacher S, Sucher R, Seehofer D. Prognostic relevance of the eighth edition of TNM classification for resected perihilar cholangiocarcinoma. J Clin Med. 2020;9(10):3152.
Farges O, Regimbeau JM, Fuks D, Le Treut YP, Cherqui D, Bachellier P, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100(2):274–83.
van Keulen AM, Buettner S, Erdmann JI, Pratschke J, Ratti F, Jarnagin WR, et al. Multivariable prediction model for both 90-day mortality and long-term survival for individual patients with perihilar cholangiocarcinoma: does the predicted survival justify the surgical risk? Br J Surg. 2023;110(5):599–605.
Franken LC, Coelen RJS, Erdmann JI, Verheij J, Kop MP, van Gulik TM, et al. Multidetector computed tomography assessment of vascular involvement in perihilar cholangiocarcinoma. Quant Imaging Med Surg. 2021;11(11):4514–21.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81.
Caillol F, Bories E, Zemmour C, Pesenti C, Ratone JP, Gilabert M, et al. Palliative endoscopic drainage of malignant stenosis of biliary confluence: Efficiency of multiple drainage approach to drain a maximum of liver segments. United European Gastroenterol J. 2019;7(1):52–9.
Casadio M, Cardinale V, Klümpen HJ, Morement H, Lacasta A, Koerkamp BG, et al. Setup of multidisciplinary team discussions for patients with cholangiocarcinoma: current practice and recommendations from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). ESMO Open. 2022;7(1):100377.
Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261–80.
Liao X, Zhang D. The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary cancer: a review and update. Arch Pathol Lab Med. 2021;145(5):543–53.
Gaspersz MP, Buettner S, Roos E, van Vugt JLA, Coelen RJS, Vugts J, et al. A preoperative prognostic model to predict surgical success in patients with perihilar cholangiocarcinoma. J Surg Oncol. 2018;118(3):469–76.
Wiggers JK, Groot Koerkamp B, Cieslak KP, Doussot A, van Klaveren D, Allen PJ, et al. J Am Coll Surg. 2016;223(2):321–31.
Groot Koerkamp B, Wiggers JK, Gonen M, Doussot A, Allen PJ, Besselink MGH, et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2015;26(9):1930–5.
Buettner S, van Vugt JLA, Gaspersz MP, Coelen RJS, Roos E, Labeur TA, et al. Survival after resection of perihilar cholangiocarcinoma in patients with lymph node metastases. HPB (Oxford). 2017;19(8):735–40.
Nooijen LE, Banales JM, de Boer MT, Braconi C, Folseraas T, Forner A, et al. Impact of positive lymph nodes and resection margin status on the overall survival of patients with resected perihilar cholangiocarcinoma: The ENSCCA Registry. Cancers (Basel). 2022;14(10):2389.
Hartog H, Ijzermans JN, van Gulik TM, Groot Koerkamp B. Resection of perihilar cholangiocarcinoma. Surg Clin North Am. 2016;96(2):247–67.
Serrablo A, Serrablo L, Alikhanov R, Tejedor L. Vascular resection in perihilar cholangiocarcinoma. Cancers (Basel). 2021;13(21):5278.
Mizuno T, Ebata T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, et al. Combined vascular resection for locally advanced perihilar cholangiocarcinoma. Ann Surg. 2022;275(2):382–90.
Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7(6):522–32.
Acknowledgment
The authors thank the patients and their families for the participation in this study, the "Asociación Española de Gastroenterología" (AEG) for the REDCap database license and training, and Dr. Ioana Riaño (Biodonostia Health Research Institute) for the support on ethical aspects of the Registry. This article is based upon work from European Horizon 2020 COST Action CA18122 European Cholangiocarcinoma Network (Euro-Cholangio-Net) supported by COST (European Cooperation in Science and Technology: www.cost.eu), in collaboration with the European Network for the Study of Cholangiocarcinoma (ENS-CCA: http://www.enscca.org/), the International Primary Sclerosing Cholangitis Study Group (iPSCSG: https://www.ipscsg.org/), the European Reference Network on Rare Liver Diseases (ERN-Rare Liver: https://rare-liver.eu/) and the European Reference Network on Rare Adult Cancers (solid tumors; EURACAN: https://euracan.eu/).
Funding
The ENS-CCA Registry is competitively funded by the European Association for the Study of the Liver (EASL; Registry grant awards 2016, 2019 and 2022) and Incyte Biosciences International Sàrl (grant award 2020). Dr Jesus M Banales and Dr Angela Lamarca received funding from the European Union’s Horizon 2020 Research and Innovation Programme [grant number 825510, ESCALON]. Dr Angela Lamarca received funding from Spanish Society of Medical Oncology (SEOM) Fellowship Programme (Return Fellowship).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Angela Lamarca declares travel and educational support from Ipsen, Pfizer, Bayer, AAA, SirtEx, Novartis, Mylan, Delcath Advanza Pharma, and Roche; speaker honoraria from Merck, Pfizer, Ipsen, Incyte, AAA, QED, Servier, Astra Zeneca, EISAI, Roche, and Advanz Pharma; advisory and consultancy honoraria from EISAI, Nutricia Ipsen, QED, Roche, Servier, Boston Scientific, Albireo Pharma, AstraZeneca, Boehringer Ingelheim, GENFIT, TransThera Biosciences, and Taiho; principal Investigator associated Institutional Funding form QED, Merck, Boehringer Ingelheim, Servier, AstraZeneca, GenFit, Albireo Pharma; she is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. Chiara Braconi receives honoraria from AstraZeneca (consultant, speaker, spouse employee), Incyte (consultant, speaker), Servier (consultant), Boehringer-Ingelheim (consultant); she receives research funds from Avacta, Medannex, and Servier. Jesús María Bañales declares research grants (from Incyte and Albireo), personal fees for lecturer (from Intercept, AstraZeneca and Incyte), and consulting role (for Albireo, Rubió Metabolomics, Ikan Biotech, and CYMABay). Dr. Juan Valle reports personal fees from Agios, personal fees from AstraZeneca, personal fees from Baxter, personal fees from Genoscience Pharma, personal fees from Hutchison Medipharma, personal fees from Imaging Equipment Ltd (AAA), personal fees from Incyte, personal fees from Ipsen, personal fees from Mundipharma EDO, personal fees from Mylan, grants, personal fees and non-financial support from NuCana, personal fees from QED, personal fees from Servier, personal fees from Sirtex, personal fees from Zymeworks, outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Keulen, AM., Buettner, S., Olthof, P.B. et al. Comparing Survival of Perihilar Cholangiocarcinoma After R1 Resection Versus Palliative Chemotherapy for Unresected Localized Disease. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-15582-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-15582-5