Abstract
Background
In 2023 alone, it’s estimated that over 64,000 patients will be diagnosed with PDAC and more than 50,000 patients will die of the disease. Current guidelines recommend neoadjuvant therapy for patients with borderline resectable and locally advanced PDAC, and data is emerging on its role in resectable disease. Neoadjuvant chemotherapy may increase the number of patients able to receive complete chemotherapy regimens, increase the rate of microscopically tumor-free resection (R0) margin, and aide in identifying unfavorable tumor biology. To date, this is the largest study to examine surgical outcomes after long-duration neoadjuvant chemotherapy for PDAC.
Methods
Retrospective analysis of single-institution data.
Results
The routine use of long-duration therapy in our study (median cycles: FOLFIRINOX = 10; gemcitabine-based = 7) is unique. The majority (85%) of patients received FOLFIRINOX without radiation therapy; the R0 resection rate was 76%. Median OS was 41 months and did not differ significantly among patients with resectable, borderline-resectable, or locally advanced disease.
Conclusions
This study demonstrates that in patients who undergo surgical resection after receipt of long-duration neoadjuvant FOLFIRINOX therapy alone, survival outcomes are similar regardless of pretreatment resectability status and that favorable surgical outcomes can be attained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic ductal adenocarcinoma (PDAC) has the highest mortality rate of all major cancers, with a 5-year survival rate of 12%.1 The American Cancer Society estimates that in 2023 alone, over 64,000 patients will be diagnosed with PDAC and more than 50,000 patients will die of the disease.2 Although surgical resection provides the only chance for cure, oncologic outcomes remain dismal. After surgery, adjuvant chemotherapy improves overall survival (OS), and multiagent chemotherapy is associated with improved outcomes relative to gemcitabine alone.3,4 However, many patients are unable to complete adjuvant chemotherapy after surgery.4
Currently the only US National Comprehensive Cancer Network (NCCN) category 1 recommendations regarding chemotherapy treatment strategies are in the use of adjuvant FOLFIRINOX or gemcitabine-based therapy.5 However, many use neoadjuvant therapy for patients with borderline resectable and locally advanced PDAC,6 and data are emerging on its role in resectable disease.7,8 In addition to possibly increasing the number of patients able to receive complete chemotherapy regimens, neoadjuvant chemotherapy is being used to treat suspected systemic disease, increase the rate of microscopically tumor-free resection margin (R0), identify unfavorable biology, and select patients who are most likely to benefit from surgery.6
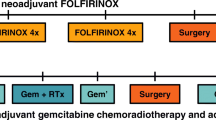
We previously reported our initial experience with long-duration neoadjuvant FOLFIRINOX (median cycles = 9) for patients (n = 26) with borderline resectable disease.9 The favorable early results led us to expand the use of long-duration neoadjuvant FOLFIRINOX to patients with resectable and locally advanced disease. We used chemotherapy alone because of the unclear added benefit of radiation therapy to chemotherapy for PDAC in the neoadjuvant and adjuvant setting.6,7,10,11,12 We now report the outcomes of 152 patients, including the 26 reported in our earlier study, treated with long-duration neoadjuvant chemotherapy (FOLFIRINOX n = 128, median cycles = 10; gemcitabine-based n = 24, median cycles = 7) with resectable (52%), borderline resectable (28%), and locally advanced (20%) disease. Because the vast majority (93%) of patients received chemotherapy only, this study provides important insight into a neoadjuvant treatment approach for PDAC that does not include radiation therapy.
Methods
Study Design and Patient Cohort
This was a retrospective analysis of a tertiary care center’s pancreatic cancer surgical database maintained at the University of California, San Francisco (UCSF). Patients were included if they had undergone surgical resection at UCSF for PDAC, received neoadjuvant chemotherapy with or without radiation therapy, and were diagnosed between January 2011 and August 2021. The electronic medical records (EMRs) of individual patients were reviewed and relevant data collected; publicly available records were reviewed to determine the completeness of mortality data. This study was approved by the Committee on Human Research at UCSF.
Pathologic Evaluation
The primary objective of this study was to determine the rate of R0 resection, defined as the absence of microscopic tumor cells within 1 mm of the surgical margins. On certain pathology reports, this information was not provided and the report of ‘negative margin’ was used (n = 8). Secondary objectives were assessments of pathologic, radiographic, and biomarker treatment responses. Pathologic treatment response, estimated by the extent of residual tumor, was graded according to the Evans grading system and was collected from pathology reports. Tumor stage was assessed according to the American Joint Committee on Cancer (AJCC) staging system for pancreatic cancer, with careful consideration for differences between the 7th and 8th edition staging.13 Histologic grade, total number of lymph nodes examined, presence of positive lymph nodes, and tumor size were recorded.
Radiologic Staging
The radiographic effect of neoadjuvant chemotherapy was determined by comparing pre- and post-neoadjuvant therapy imaging. All abdominal multiphasic pancreatic protocol computed tomography (CT) scans obtained at baseline and after chemotherapy and chemotherapy/radiation were reviewed by an experienced surgical oncologist and a radiologist. Tumor location at baseline was recorded. Resectability status, as defined by the NCCN definitions, was used to categorize patients as resectable, borderline resectable (BRPC) and locally advanced (LAPC).14,15 In our study, arterial and venous involvement with <180° tumor contact of the vessel circumference was referred to as abutment, and >180° tumor contact was described as encasement. In the case of venous involvement, the presence of vessel narrowing or occlusion was also assessed. Because radiographic measurements according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria can be challenging for primary pancreatic tumors,16 treatment response was qualitatively classified into one of three categories: shrinkage, stable, or progression. Shrinkage was defined as less vascular involvement, evidence of a new fat plane between the tumor and vessel, or decreased primary tumor size; stable was characterized as absence of change in either the primary tumor size or the degree of vascular involvement; and progression was defined as an increase in vascular involvement, new obliteration of a fat plane, and/or increased primary tumor size.
Neoadjuvant Therapy
Use of and response to neoadjuvant therapy alone (either FOLFIRINOX or gemcitabine-based therapy) or neoadjuvant therapy with radiation therapy were recorded, as were the number of treatment cycles. The total amount of chemotherapy administered was estimated based on the number of treatment cycles reported. One cycle of gemcitabine was a 4-week course with 3 weeks of treatment and 1 week of rest, and of FOLFIRINOX a 2-week course.17
Surgical Resection
Additional study variables included the type of operation, the need for vascular reconstruction, neoadjuvant treatment-related toxicities, perioperative complication rates, frequency and patterns of tumor recurrence, 90-day survival, disease-free survival (DFS), and OS. The Clavien–Dindo complication classification system was used to grade each 30-day surgical complication.18 DFS was defined as the time from the date of the first cycle of neoadjuvant chemotherapy until the earliest date of documented disease recurrence or death, and OS was specified as the time from the date of the first cycle of neoadjuvant chemotherapy until the date of death from any cause. Mortality data were collected primarily via EMR review, and publicly available records were reviewed when EMR data were not readily available.
Statistical Analysis
The Chi-square tests and Mann–Whitney U tests were applied to categorical and continuous variables, respectively. DFS and OS analyses were performed using the Kaplan–Meier method and descriptive statistics were applied to summarize and tabulate the data.
Results
A total of 152 patients who met the study criteria were identified, of whom 120 received neoadjuvant FOLFIRINOX chemotherapy alone, 8 received neoadjuvant FOLFIRINOX followed by chemoradiation, 22 received gemcitabine-based neoadjuvant therapy alone, and 2 received gemcitabine-based neoadjuvant therapy followed by radiation. Baseline demographic and clinical characteristics are shown in Table 1.
According to pretreatment abdominal CT scans, the most common pretreatment tumor location was in the head of the pancreas (n = 86, 57%) followed by the neck (n = 21, 14%). The most commonly involved vessels were abutment of the superior mesenteric vein (SMV; n = 81, 53%), superior mesenteric artery (SMA; n = 24, 16%), portal vein (PV; n = 17, 11%), and encasement of the common hepatic artery (CHA; n = 12, 8%) [Table 1]. When classified by resectability status according to NCCN definitions, most patients who received neoadjuvant chemotherapy alone met formal radiographic criteria for resectable disease (n = 81) or BRPC (n = 48). For patients with LAPC, FOLFIRINOX alone was the most common regimen (n = 15). For the neoadjuvant FOLFIRINOX with radiation cohort, most tumors were classified as BRPC (n = 3) or LAPC (n = 4) and only one was resectable. Median pretreatment CA19-9 was 181 (range 40–648) and did not vary significantly among neoadjuvant modalities or radiation and non-radiation groups.
The median number of treatment cycles was 10 (range 7–12) for the FOLFIRINOX-alone group and seven (range 6.5–7.5) for the gemcitabine-based-alone group. Overall, 48% of patients showed disease shrinkage and 48% had stable disease on follow-up CT scans (Table 2). Disease shrinkage occurred in three of the eight patients who received FOLFIRONIX and radiation treatment, and one of the two patients who received gemcitabine and radiation treatment.
Disease progression occurred in just five patients during neoadjuvant chemotherapy: four in the combined FOLFIRINOX groups and one in the gemcitabine with radiation group. Progression was usually locoregional. Tumor growth resulted in SMA encasement in one patient, SMA and SMV encasement in another, and both PV and SMV encasement in a third (compared with abutment in preoperative scans for all three cases). The fourth patient, whose tumor encased both the CHA and SMV at baseline, received eight cycles of gemcitabine-based neoadjuvant therapy, but the tumor progressed and encased the celiac artery. In the fifth patient, the tumor grew in size but did not encase any vessels. In two of the five cases, an R0 resection was achieved.
Neoadjuvant treatment was shortened or stopped for 40 patients (26.3%) due to various complications, including nausea (22%, n = 9), diarrhea and weight loss (15%, n = 6), and peripheral neuropathy (1%, n = 4). One patient developed hyperbilirubinemia, another developed a hypersensitivity reaction, and another patient developed suppurative cholangitis due to an occluded biliary stent, among others.
Pylorus-preserving pancreaticoduodenectomy was the most common operation performed (49%) (Table 3). Vascular reconstruction was required in 43% of all patients and 47% of those in the FOLFIRINOX-alone group. For all patients in the study group, the 30-day complication rate was 64%, including delayed gastric emptying, wound dehiscence, intra-abdominal abscess, pancreatic fistula, deep vein thrombosis, hypovolemic shock, and urinary tract infection. Clavien–Dindo distribution of grades II, I, and IIIb accounted for 42%, 21%, and 16% of total complications, respectively. Two patients (1.3%) died within 30 days and seven (4.6%) within 90 days postoperatively.
As shown in Table 4, R0 resection was achieved for 74% of all patients and 76% of patients in the FOLFIRINOX-alone group. Of those who received FOLFIRINOX alone, 43% had lymph node involvement and a median of 21 (range 15–27) lymph nodes were examined. The most common AJCC pathologic stages were yIIB (31%) and yIA (18%).
Ten percent (n = 14) of all patients had a treatment response of Evans grade III, corresponding to < 10% of residual tumor cells. Six patients (4%) had a complete response, described as AJCC stage 0 and Evans grade IV (no viable tumor). Fifty percent of patients had no nodal metastasis with AJCC staging between yIA and yIIA.
The overall recurrence rate of the entire cohort was 45% (Table 5). The median OS for patients who received FOLFIRINOX with or without radiation was 41 months (Fig. 1) and DFS was 38 months (Fig. 2). For patients who received gemcitabine-based therapy with and without radiation, neither the median OS nor median DFS were reached. No difference was seen in OS between patients who received FOLFIRINOX and gemcitabine (Figs. 1, 2). The median OS for resectable disease was 37 months, 45 months for BRPC disease, and 40 months for LAPC (Fig. 3) Median OS and DFS did not significantly differ among patients with resectable disease, BRPC, and LAPC, however BRPC trended towards longer OS and DFS (Fig. 3, 4).
DFS of patients who underwent FOLFIRINOX alone (no radiation therapy) stratified by resectability status. The median DFS for patients with resectable, borderline resectable, and locally advanced disease was 31 months, 41 months, and 30 months, respectively, and the relative hazard ratio was 1.1, 0.74, and 1.5 for resectable, borderline resectable, and locally advanced disease, respectively. DFS disease-free survival
Discussion
To date, this is the largest study to examine surgical outcomes after long-duration neoadjuvant chemotherapy for PDAC. Although neoadjuvant therapy is commonly used for PDAC, the routine use of long-duration therapy (FOLFIRINOX, median cycles = 10; gemcitabine-based, median cycles = 7) is unique. We found that FOLFIRINOX is associated with favorable outcomes for patients with resectable disease, BRPC, and LAPC.
In our study, patients who received long-duration neoadjuvant FOLFIRINOX and underwent surgery had a median OS of 41 months, which compares favorably with other reported results. In the SWOG1505 study, patients with resectable PDAC were randomized to six cycles of neoadjuvant FOLFIRINOX versus three cycles of neoadjuvant gemcitabine/nab-paclitaxel, a median OS of 23.2 months was reported for the perioperative FOLFIRINOX arm.19 In the Alliance A021501 trial, where patients received eight cycles of neoadjuvant FOLFIRINOX for BRPC, the median OS was 29.8 months.10 However, a higher median OS of 54.4 months was reported in the adjuvant FOLFIRINOX arm (12 cycles) of the PRODIGE-24 study; this arm comprised a highly select patient group (patients ≤79 years of age, a WHO performance score of 0 or 1, no significant cardiovascular disease, and a serum CA19-9 level of < 180 kU/L).4
Our institutional approach of using long-duration neoadjuvant chemotherapy is based on favorable early experience9 and the unclear added benefit of radiotherapy to chemotherapy for PDAC in the neoadjuvant and adjuvant setting. Results from several randomized trials suggest that radiotherapy provides no added benefit to chemotherapy in the neoadjuvant setting. In the Dutch randomized phase III PREOPANC trial, patients with resectable disease and BRPC were randomized to neoadjuvant chemoradiation versus upfront surgery, and there was no significant difference in OS.7 In the Alliance A021501 phase II trial, patients with BRPC were randomized to neoadjuvant FOLFIRINOX with or without radiotherapy followed by four cycles of postoperative FOLFOX6. The median OS was 29.8 months for the chemotherapy-only arm and 17.1 months for the chemotherapy plus preoperative radiotherapy arm.10 In the ESPAC5 phase II trial, patients with BRPC who received neoadjuvant chemotherapy (FOLFIRINOX or gemcitabine) had better 1-year OS than patients who had upfront surgery; however, those who received neoadjuvant chemotherapy with radiation did not.11 Finally, for patients with LAPC, the CONKO-007 phase III randomized trial examined the addition of radiotherapy to chemotherapy and failed to meet the primary endpoint of R0 resection rate, although the authors did report an improved resection rate without a concomitant improvement in OS.12
Although current guidelines recommend neoadjuvant therapy for patients with BRPC or LAPC, the role of neoadjuvant therapy for resectable disease is evolving. In the SWOG1505 phase II trial, there were no differences in 2-year OS (47% vs. 48%) or median OS (23.2 months vs. 23.6 months).8 In the SWOG1505 trial, 49% of patients completed all therapy, mainly due to the inability to receive adjuvant chemotherapy, and 88% of patients were able to complete neoadjuvant therapy.8 Based on these findings and the PRODIGE-24 study4 in which only two-thirds of patients completed adjuvant chemotherapy, the authors raised the question of whether total neoadjuvant therapy should be considered in future clinical trials.8 Our findings support this strategy; our patients completed a median of 10 cycles of FOLFIRINOX prior to surgery.
Recently, a large retrospective study using the National Cancer Database found that for patients with resectable PDAC, multiagent neoadjuvant chemotherapy followed by resection was associated with better survival than upfront surgery.20 A recent meta-analysis comparing upfront resection with neoadjuvant therapy in resectable pancreatic cancer did not find an improvement in DFS or OS.21 In the ongoing Alliance A021806 phase III trial, patients with resectable disease were randomized to eight cycles of neoadjuvant FOLFIRINOX followed by four cycles of adjuvant FOLFIRINOX versus 12 cycles of adjuvant FOLFIRINOX. The primary endpoint of this trial was two-year OS.22 While we await the results of this trial, the findings from our study provide insight into the favorable outcomes of long-duration neoadjuvant FOLFIRINOX for PDAC.
Our study has some limitations. First, because this was a retrospective analysis from a single-institution, our study is subject to referral and selection bias. All patients included in this study underwent surgery, so this is a highly selected group. Second, because the total number of patients who received long-duration neoadjuvant chemotherapy is not known, we could not calculate the resection rate. On the other hand, an important strength of our study was the ability to capture granular data regarding clinicopathological features that may not be available in large databases.
Conclusion
Although neoadjuvant therapy is commonly used for PDAC, the routine use of long-duration therapy (median cycles: FOLFIRINOX = 10; gemcitabine-based = 7) is unique. Most (85%) patients received FOLFIRINOX; the R0 resection rate was 76%. Median OS was 41 months and did not differ significantly among patients with resectable disease, BRPC, or LAPC. This study shows that favorable surgical outcomes can be attained after long-duration neoadjuvant FOLFIRINOX therapy alone.
References
Cancer Facts & Figures 2023|American Cancer Society. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html. Accessed 30 Jun 2023.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. https://doi.org/10.3322/caac.21763.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–24. https://doi.org/10.1016/S0140-6736(16)32409-6.
Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–406. https://doi.org/10.1056/NEJMoa1809775.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Available at: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
Springfeld C, Ferrone CR, Katz MHG, et al. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol. 2023;20(5):318–37. https://doi.org/10.1038/s41571-023-00746-1.
Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763–73. https://doi.org/10.1200/JCO.19.02274.
Sohal DPS, Duong M, Ahmad SA, et al. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2021;7(3):421–7. https://doi.org/10.1001/jamaoncol.2020.7328.
Kim SS, Nakakura EK, Wang ZJ, et al. Preoperative FOLFIRINOX for borderline resectable pancreatic cancer: is radiation necessary in the modern era of chemotherapy? J Surg Oncol. 2016;114(5):587–96. https://doi.org/10.1002/jso.24375.
Katz MHG, Shi Q, Meyers J, et al. Efficacy of preoperative mFOLFIRINOX vs mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: The A021501 phase 2 randomized clinical trial. JAMA Oncol. 2022;8(9):1263–70. https://doi.org/10.1001/jamaoncol.2022.2319.
Ghaneh P, Palmer D, Cicconi S, et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(2):157–68. https://doi.org/10.1016/S2468-1253(22)00348-X.
Fietkau R, Ghadimi M, Grützmann R, et al. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresectable locally advanced pancreatic cancer: first results of the CONKO-007 trial. JCO. 2022;40(16 Suppl):4008. https://doi.org/10.1200/JCO.2022.40.16_suppl.4008.
Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol. 2017;24(7):2023–30. https://doi.org/10.1245/s10434-017-5810-x.
Guidelines Detail. NCCN. Available at: https://www.nccn.org/guidelines/guidelines-detail. Accessed 30 Jun 2023.
Kulkarni NM, Soloff EV, Tolat PP, et al. White paper on pancreatic ductal adenocarcinoma from society of abdominal radiology’s disease-focused panel for pancreatic ductal adenocarcinoma: part I, AJCC staging system, NCCN guidelines, and borderline resectable disease. Abdom Radiol. 2020;45(3):716–28. https://doi.org/10.1007/s00261-019-02289-5.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. https://doi.org/10.1056/NEJMoa1011923.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Ahmad SA, Duong M, Sohal DPS, et al. Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX vs. gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann Surg. 2020;272(3):481–6. https://doi.org/10.1097/SLA.0000000000004155.
Sugawara T, Rodriguez Franco S, Sherman S, et al. Association of adjuvant chemotherapy in patients with resected pancreatic adenocarcinoma after multiagent neoadjuvant chemotherapy. JAMA Oncology. 2023;9(3):316–23. https://doi.org/10.1001/jamaoncol.2022.5808.
Junior PLSU, Silva DD, de Castro NM, et al. Does neoadjuvant treatment in resectable pancreatic cancer improve overall survival? A systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2023. https://doi.org/10.1016/j.esmoop.2022.100771.
Chawla A, Ferrone CR. Neoadjuvant therapy for resectable pancreatic cancer: an evolving paradigm shift. Frontiers in Oncology. 2019;9. Available at: https://www.frontiersin.org/article/https://doi.org/10.3389/fonc.2019.01085. Accessed 26 Jan 2022.
Acknowledgment
Ms. Pamela Derish, MA reviewed this manuscript and provided assistance with finalizing this paper. Dr. Fei Jiang and the UCSF Clinical and Translational Science Institute provided technical assistance with statistical analysis and OS/DFS curves.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Margaret Tempero has held consultancy roles for Astellas Pharma Global Development Inc. (DSMC), AstraZeneca, Bio Ascend-B2ACT Therapeutics Genex, BioSapiens, ElmediX, Debiopharm, Geistlich Pharma, IPSEN Biopharma, Inc., Mirati Therapeutics Inc., Novartis Pharmaceuticals, Oncolytics, Global Bio Access Fund, SAB Peptomyc, S.L., and Geron Corporation; advisory board roles for BeiGene, Biotech Research, Boehringer Ingelheim, Global Bio Access Fund, Merus, and BMS; and served as a Scientific Advisor for Exact Sciences. Phoebe N. Miller, Fernanda Romero-Hernandez, Lucia Calthorpe, Jaeyun Jane Wang, Sunhee S. Kim, Carlos U. Corvera, Kenzo Hirose, Kimberly S. Kirkwood, Ryutaro Hirose, Ajay V. Maker, Adnan A. Alseidi, Mohamed A. Adam, Grace E. Kim, Andrew H. Ko, and Eric K. Nakakura have no conflicts of interest or funding to declare in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miller, P.N., Romero-Hernandez, F., Calthorpe, L. et al. Long-Duration Neoadjuvant Therapy with FOLFIRINOX Yields Favorable Outcomes for Patients Who Undergo Surgery for Pancreatic Cancer. Ann Surg Oncol 31, 6147–6156 (2024). https://doi.org/10.1245/s10434-024-15579-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15579-0