Abstract
Background
Radical esophagectomy for resectable esophageal cancer is a major surgical intervention, associated with considerable postoperative morbidity. The introduction of robotic surgical platforms in esophagectomy may enhance advantages of minimally invasive surgery enabled by laparoscopy and thoracoscopy, including reduced postoperative pain and pulmonary complications. This systematic review aims to assess the clinical and oncological benefits of robot-assisted esophagectomy.
Methods
A systematic literature search of the MEDLINE (PubMed), Embase and Cochrane databases was performed for studies published up to 1 August 2023. This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocols and was registered in the PROSPERO database (CRD42022370983). Clinical and oncological outcomes data were extracted following full-text review of eligible studies.
Results
A total of 113 studies (n = 14,701 patients, n = 2455 female) were included. The majority of the studies were retrospective in nature (n = 89, 79%), and cohort studies were the most common type of study design (n = 88, 79%). The median number of patients per study was 54. Sixty-three studies reported using a robotic surgical platform for both the abdominal and thoracic phases of the procedure. The weighted mean incidence of postoperative pneumonia was 11%, anastomotic leak 10%, total length of hospitalisation 15.2 days, and a resection margin clear of the tumour was achieved in 95% of cases.
Conclusions
There are numerous reported advantages of robot-assisted surgery for resectable esophageal cancer. A correlation between procedural volume and improvements in outcomes with robotic esophagectomy has also been identified. Multicentre comparative clinical studies are essential to identify the true objective benefit on outcomes compared with conventional surgical approaches before robotic surgery is accepted as standard of practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Surgical resection is a key component of curative management of esophageal cancer, the seventh most common cancer worldwide, and is associated with significant morbidity and mortality.1 Neoadjuvant chemo(radio)therapy provides a survival advantage over surgery alone, with 5 year survival rates of up to 50%.2 The physical trauma of open esophagectomy with associated postoperative morbidity has considerable impact on survival and health-related quality of life (QOL).3,4,5
Minimally invasive surgery (MIS) confers several benefits to patients with resectable esophageal cancer, with multiple trials comparing outcomes with open surgery.6,7,9 Advantages include reduced postoperative pain due to smaller incisions, lower incidence of pneumonia, and earlier mobilisation, without impacting overall survival (OS) and disease-free survival (DFS).10,11,12 However, evidence suggests that open esophagectomy is associated with shorter operative time but equivalent oncological outcomes to MIS.13
Robotic surgical platforms seek to improve perioperative outcomes and enhance what can be achieved with conventional MIS.14 The three-dimensional view and articulated instruments afforded by the robotic platform can enhance dissection around difficult planes and improve surgeons’ views.15 Robotic surgery is popular in colorectal surgery and gynaecology, and is the gold standard for prostatic resection.16
The first reported robot-assisted esophagectomy, using the daVinci telemanipulator instrument (Intuitive Surgical, Mountainview, CA, USA), was published by Melvin et al.17 in 2002. Since then, the market for robotic surgical platforms has expanded with numerous systems, including the HugoTM (Medtronic, Minneapolis, MN, USA) and Versius (CMR Surgical Ltd, Cambridge, UK).
Although the number of robotic esophagectomies has increased worldwide, this procedure is not considered standard treatment for resectable esophageal cancer due to high costs and limited high-level evidence supporting its use.18 Current practice may incorporate open surgery and MIS into a ‘hybrid’ procedure. For example, laparoscopy is used for the abdominal phase and an open thoracotomy is used for the chest phase.19 This affords patients some of the benefits of MIS, particularly regarding pain and length of hospitalisation.
The primary aim of this systematic literature review is to assess clinical and oncological outcomes of robot-assisted esophagectomy. We describe current trends in practice, evaluate the advantages and disadvantages conferred by the robotic surgical platform, and elucidate evidence of a learning curve among centres who have recently adopted this technique for resectable esophageal cancer.
Methods
Search Strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) protocols in observational studies and randomised trials,19 and was registered on the international prospective register of systematic reviews (PROSPERO), registration number CRD42022370983. A review protocol was not prepared.
A search of the MEDLINE (PubMed), Embase and Cochrane databases was performed by two authors (NMP and PHP), identifying all studies published up to 1 August 2023. The Medical Subject Heading (MeSH) terms ‘robotic surgery’, ‘minimally invasive surgery’, ‘esophageal cancer’, and ‘outcomes’ were included. Conference proceedings and articles not published in English were excluded.
Data Extraction
Two reviewers (NMP and PHP) screened articles independently by title and abstract before reading the full text of eligible studies. Relevant data including demographics and parameters on perioperative outcomes, including lymph node yield (LNY), anastomotic leak (AL) rate and length of stay (LoS), were collated.
Statistical Analysis
Single-arm meta-analyses of oncological and clinical outcomes were performed using RStudio version 4.3.2 (Boston, MA, USA) [Table 1].20 Weighted mean (95% confidence interval) and heterogeneity (I2) were calculated for all studies. Statistical significance was confirmed at p < 0.05. Forest plots were constructed for all outcomes, examples of which are demonstrated in Figs. 1, 2 and 3.
Bias Assessment
Risk of bias was assessed using the modified Newcastle–Ottawa scale for non-randomised studies, and the modified Jadad scale for randomised trials.21,22
Results
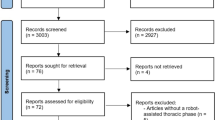
The initial literature search yielded 2192 studies. Following screening for full-text eligibility, 113 studies (n = 14,701 patients) were included (Fig. 4).
PRISMA reporting standards.23 PRISMA preferred reporting items for systematic reviews and meta-analyses
Cohort studies reporting on retrospectively collected data were the most common type of study. Four (4%) clinical trials on outcomes following robotic esophagectomy were included. The median number of patients per study was 54, with a median age of 64 years. The most common esophageal malignancy was adenocarcinoma (54%) [Table 1]. Other esophageal malignancies, including gastrointestinal (GI) stromal tumours, were grouped under ‘other malignancy’ (Fig. 5).
Among the included studies, the robotic platform was most commonly used in the thoracic phase (85 studies, 75%) of esophageal cancer resections (Tables 2 and 3). In the abdominal phase, the robotic approach was the most popular (64 studies, 57%), and conventional laparoscopy was used in 20 studies (18%) (Tables 2 and 3). Thirty-one studies (27%) confirmed use of a robotic platform in one phase but did not classify the approach used for others. Most studies reported two-stage procedures (90 studies, 80%) and six reported a transhiatal approach (5%).
Preoperative tumour staging was not presented by the studies. Weighted mean incidence of oncological and postoperative outcomes are presented in Table 4. Use of postoperative opioid analgesia was reported by five studies (4%) [Table 5].
Neoadjuvant chemoradiation (70 studies, 62%) was the most frequently used perioperative treatment, followed by neoadjuvant chemotherapy (48 studies, 43%). Use of adjuvant therapy was reported in 14 studies (12.4%).
Discussion
In this systematic review, we present clinical and oncological outcomes of elective robotic esophagectomy for esophageal cancer. Robotic esophagectomy is a relatively modern modality with variable uptake worldwide. This may contribute to the heterogeneity in results, especially from units with varying surgical experience.
Type of Study
Retrospective cohort (79%) was the most common study type, with four clinical trials eligible for inclusion.1,24,25,26 Several trials comparing robotic esophagectomy with open and conventional MIS are awaiting publication of the results;27,28,29 therefore, limited data on perioperative outcomes are currently available. Most studies were from North American (30%), and Chinese centres (20%). The wider adoption of robotic surgery throughout the United States reflects greater availability of robotic platforms and supporting infrastructure.30 Furthermore, China accounts for nearly half the global disease burden of esophageal squamous cell carcinoma (ESCC), enabling centres to undertake more resections compared with the West.31,32
Surgical Approach
Eighty-five (75%) studies reported a robot-assisted thoracic phase, with 64 studies (57%) performing both robotic abdominal and thoracic phases. A hybrid minimally invasive approach involving laparoscopic abdominal and robotic thoracic phases was reported in 19 studies (17%).1,27,33 Open thoracic or abdominal phases were used in combination with robotic surgery in five studies (4%).34,35,36,37,38 Initially, the literature reported equivalent oncological outcomes and shorter procedure length in open esophagectomy when compared with thoraco-laparoscopic approaches.13 Therefore, many surgeons may lack experience in thoraco-laparoscopic esophageal resection, moving immediately to the robotic console without developing skills in what may be perceived as an ‘intermediate step’ in MIS.14,34
Three studies reinforce the notion of learning curves associated with developing proficiency with novel surgical technologies, manifested by analysing learning curves in robotic esophagectomy.39,40,41 These identified the mean number of cases required before surgeons experienced significant improvements in outcomes. Park et al. suggested a change point of 28 robot-assisted esophagectomies for an observed increase in LNY from 25 to 45 (p < 0.001);39 however, other factors, including marked reduction in the incidence of complications, for example reduction in AL rate, were reported after 80 and 85 cases, respectively. These findings are supported by the cumulative sum (CUSUM) learning curves derived by Kingma et al., where 22 robotic esophagectomy cases were performed before a plateau in estimated blood loss (EBL) and operative time was noticed, suggesting certain components of the procedure take a greater number of cases for expert credentialing.40
Operative Time
Robotic esophagectomy has been associated with longer operating times than open surgery.1 This is partly due to time spent ‘docking’ instruments, requiring familiarisation of theatre teams with the robotic platform. This literature review reported a weighted mean operative time of 372.16 min (range 168–808 min) for robotic esophageal cancer resections, taken as the total operating time and not solely time spent on the robotic console.
Kingma et al. identified that after 23 cases, surgeons noticed a reduction in operating time for both the thoracic and abdominal phases, plateauing at case number 70.40 Park et al. confirmed that temporal improvement is seen with accumulated experience, but this occurred after 80 cases.39 It can be hypothesised that with greater experience comes reduced operating times, reiterating the presence of a learning curve. With sufficient experience, centres may then be able to match higher-volume American and Chinese units.42,43,44,45,46,47,48,49
Perioperative Complications and Length of Stay
An advantage of the robotic platform is the ability to perform finer dissection within challenging anatomical areas, with reduced EBL and rate of visceral injury.50 Minimising these complications may allow for shorter recovery times and reduced length of hospitalisation.
Critical Care and Total Inpatient Length of Stay, Postoperative Pneumonia and Enhanced Recovery After Surgery
There is a significant, multifactorial physiological stress response to major surgery, and open esophagectomy has a significant impact on patients.5 The degree of postoperative haemodynamic and respiratory support required typically results in admitting patients to Level 1 care postoperatively.51
Weighted mean critical care and total inpatient LoS for the included studies were 1.92 days (range 0.85–23) and 15.2 days (range 7–24), respectively. This indicates significant variation among units, which may be associated with perioperative complications. In their single-centre cohort study of 321 patients, Angehern et al. reported shorter duration of hospitalisation (18.5 days) among their open esophagectomy cohort compared with those who had robotic procedures (20 days, p = 0.368).34 This contradicts the notion that MIS is associated with shorter LoS. However, given that this is a novel surgical technology, the surgeons may have felt inclined to keep patients in under observation for longer, in anticipation of delayed postoperative complications. This is despite reduced rates of re-intervention among the robotic cohort (5.3%) compared with patients who had open surgery (7.9%).34 Pneumonia is a common cause of morbidity after esophagectomy and poor pain control is a major causative factor.42,52 Smaller incisions required in robotic esophagectomy and the reduced nerve injury result in less pain after surgery, better respiratory effort and reduced risk of pneumonia. Tsunoda et al. demonstrated a lower rate of pulmonary complications (18%, p = 0.006) among patients who underwent robot-assisted esophagectomy compared with conventional minimally invasive esophagectomy (44%).53 The incidence of postoperative pneumonia ranged from 0% to 45.4%; however, the literature varied in its definition and criteria influencing treatment decisions.42 Notably, Meredith et al. reported no significant difference in the incidence of pneumonia between patients undergoing open, robotic or conventional minimally invasive esophagectomy.52 In comparison, three studies reported an incidence of postoperative pneumonia of >30%, despite all patients undergoing laparoscopic and robotic phases, suggesting a multifactorial aetiology for postoperative pneumonia.33,54,55
Twenty studies reported total inpatient LoS of < 10 days; none of these studies used an open approach for the thoracic or abdominal phases.25,41,43,44,52,56,57,58,59,60,61,62,63,64,65,66,67,68,69 In comparison, 27 studies reported total LoS > 14 days, of which six used an open approach in either the abdominal or thoracic phases.5,24,25,33,42,45,46,55,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85 This indicates the robotic and conventional minimally invasive approaches are associated with shorter LoS, however clinical trials are required to validate this.1,26,27
Factors contributing to reduced LoS include less postoperative pain and nausea, earlier introduction of oral intake, and mobilisation.5,81,86 Enhanced Recovery After Surgery (ERAS) programmes or ‘fast-track protocols’ in elective upper GI resection have led to improvements in patient outcomes, including LoS and postoperative morbidity, by implementing a standardised pathway for patients and care providers.87,88 No studies commented on the use of ERAS. Since robotic esophagectomy is a recent adoption for many units, surgeons may implement a tailored postoperative recovery programme instead of a goal-orientated ERAS pathway.89
Twenty-eight studies reported on rate of reoperation, ranging from 0 to 35%. This may reflect varying levels of experience with robotic esophagectomy, and may also be explained by the availability of endoscopy and interventional radiology, which could be used as alternatives to manage selected complications.28 Of note, when comparing the open approach with all minimally invasive approaches, the rates of return to theatre did not differ significantly.34,52,61
Additional comparative perioperative measures, including time to mobilisation, quantitative data on postoperative pain, and hospital readmission, would be beneficial to describe tangible representative outcomes across studies.
Blood Loss
Weighted mean EBL across the included studies was 197.7 mLs (range 35–598 mLs), however blood loss per operative phase was not specified. The two studies with the lowest mean blood loss, 35 mLs in total, are also the two where a totally minimally invasive esophagectomy was performed.63,78 These studies highlight key advantages offered by MIS through smaller incisions and reduced surgical trauma, giving robotic surgery the advantage over open approaches in resectable esophageal cancer.
Oncological Outcomes and Perioperative Therapy
Negative resection margins (R0) and LNY were collated to assess perioperative oncological outcomes of the included studies. Aside from reduced postoperative pain and shorter LoS, local disease control must be achieved to potentially improve OS and reduce the chances of recurrence. Although resection margin involvement in the surgical specimen was reported by 87 studies (77.0%), it was not specified whether this related to longitudinal or circumferential margins (CRMs). Median positive margin status from the included studies was 3.48%, demonstrating high rates of ‘curative’ resection were achieved with robotic surgery. In comparison, six studies reported positive resection margin rates of 10% or higher.40,56,61,90,91,92
Comparisons with national registries should be performed for contextualisation. The UK National Oesophago-Gastric Cancer Audit reported a 4.2% positive longitudinal and 20.3% positive CRM status for all esophageal resections performed from April 2018 to May 2021.93 Eight years of data from the Dutch Upper Gastrointestinal Cancer Audit (DUCA) reported a positive CRM rate ranging from 3.7 to 6.8%, noting the higher utilisation of neoadjuvant chemoradiotherapy, and 8.7% in Swedish registries.51,94 The higher R0 rate reported in this review compared with contemporary registry data, suggests the technical benefits offered by the robotic platform may contribute to greater R0 rates by improving dissection in difficult anatomical locations.50 However, other factors, including access to neoadjuvant therapies and disease stage at presentation, may also impact on achieving clear resection margins.
Furthermore, case selection may influence reported outcomes, especially for centres new to performing robot-assisted esophagectomy. Less complex cases may be chosen when testing a novel technique, which may influence outcomes, including R0 resection rate.
None of the included studies reported on CRM status in the resected esophageal specimens. The literature has highlighted the importance of CRM as an independent prognostic factor for local disease recurrence and survival in esophageal cancer.95,96,97,98 Although the literature suggests that robotic platforms can improve perioperative outcomes, including pulmonary complications and LoS, oncological outcomes are crucial to improving survival for potentially curative disease and should be recorded as standard practice.36
Standardised lymphadenectomy is a key component of esophagectomy for accurate disease staging, local disease control and prognostication for OS.99 Current guidelines indicate at least 15 LNs must be submitted for pathological examination according to the National Comprehensive Cancer Network (NCCN) and National Oesophago-Gastric Cancer Audit (NOGCA);93,100,101 however, standards of and experience in histopathological analysis may vary between centres. This is reflected by the studies included in this review, with a range in LNY of 8–69 nodes, despite a median of 25 nodes. This suggests a significant variability in the extent of lymphadenectomy performed in robotic esophagectomy. Four studies have highlighted that the quality of lymphadenectomy in thoracoscopic esophageal resection is inferior to robotic surgery or thoracotomy, which may explain why uptake of thoracoscopy in the esophagectomy is limited, especially now that robotic surgery is increasingly available.73,74,75,82
Three studies reported significantly lower LNY than the median and the recommended minimum.14,102,103 Of these, Washington et al. also reported a positive resection margin rate of 5.56%, above the median of 3.48%.14 Furthermore, they reported equivalent LNY when comparing laparoscopic (13.9) and robotic (14.3) esophagectomy.14 This was corroborated by Zhang et al., i.e. 19.1 nodes during thoraco-laparoscopic McKeown esophagectomy compared with 19.3 in robotic.58 This highlights the importance of following key principles of oncological surgery. In particular, that quality of lymphadenectomy should not be compromised when using a novel surgical technology, even though said new technology may offer other benefits to patients.
Factors influencing the use of oncological therapies include prevalence of different tumour types and recognised standard of care among units. This systematic review highlights international variation in practice, for example, giving definitive chemoradiotherapy in ESCC followed by salvage esophagectomy, versus neoadjuvant chemotherapy followed by surgery.26,36,46,54,67,70,82,91,92,101
The published literature supports the use of adjuvant therapy after neoadjuvant treatment and esophagectomy with clear resection margins, citing an improved OS up to 5 years.104,105 However, just 14 studies (12%, n = 380 patients) reported giving adjuvant therapy. Perioperative therapy, followed by a radical robotic esophagectomy with clear resection margins, without postoperative complications, may enable patients to proceed on to complete adjuvant therapy, improving OS and RFS.105,106
Anastomotic Leak and Chyle Leak
Reported morbidity in esophagectomy can be as high as 50%, with AL and chyle leak (CL) responsible for the greatest risk of prolonged hospitalisation and mortality.49,107 Weighted mean reported AL and CL rates were 10% and 4%, respectively.
Fifty-one studies (45%) specified the type of anastomosis created when reporting AL rates. However, AL rate did not vary considerably between circular (8.55%), linear stapled (8.75%) or hand-sewn (8.6%) anastomoses. Six studies reported performing either a robot-assisted hand-sewn or stapled intrathoracic esophagogastric anastomosis, with a leak rate ranging from 0 to 16%.58,66,90,108,109,110 Five studies were carried out in American and Chinese institutions; both were associated with more experience in robotic esophagectomy, which may explain their lower AL rates. In comparison with established national registries, the DUCA reported incidence ranging from 18.2 to 19.3% for all intrathoracic and cervical anastomoses, regardless of technique, and the UK Oesophago-Gastric Anastomosis Audit (OGAA) reported rates of 12.2% and 20.1% for intrathoracic and cervical anastomoses, respectively.51,111 As with other outcomes of robotic esophagectomy, volume and experience in performance of the procedural steps directly influence outcomes.
Although most studies reported performing a hand-sewn or stapled extracorporeal anastomosis, there was no appreciable difference in the AL rate between the two subgroups.38,103,112 Exteriorising the proximal esophagus and gastric conduit to form a hand-sewn or stapled anastomosis remains the preferred means of restoring continuity as it is technically less challenging than an anastomosis formed entirely within the body cavity through minimally invasive approaches.83 Follow-up data from the included studies did not report the incidence of anastomotic strictures and therefore it was not possible to make further comparisons between techniques.
Incidence of CL was reported by 72 studies (64%). Although the average reported rates ranged from 1.1 to 3.8%, the incidence of CL among the included studies was as high as 29%.33,107 As suggested by Dezube et al., experience may be the determining factor influencing the risk of CL in esophagectomy. As such, this may be an important parameter to assess for competence in performing robotic esophagectomy in learning curve analyses alongside parameters including operative time.39,107 The ramifications of a persistent CL are associated with infection, electrolyte imbalance, hypovolaemia, and nutritional derangement, causing prolonged hospitalisation, delayed oral intake and impact on QOL.
Comparison of Two- and Three-Stage Robotic Esophagectomy
A total of 51 and 10 studies reported on two- and three-stage esophagectomy using a robotic platform for both abdominal and thoracic phases, respectively. More cases of ESCC were managed with three-stage esophagectomy (25 cases) than two-stage (21 cases), consistent with the preponderance of SCC in the proximal esophagus.113 Table 6 highlights that robotic three-stage esophagectomy was associated with longer average operating time, and greater blood loss and AL rate compared with two-stage procedures. The addition of a third phase may explain the prolonged time taken to perform this resection and the associated higher blood loss.
A robotic cervical phase was reported in four studies, compared with six studies performing an open lymphadenectomy and anastomosis. GI surgeons may begin developing robotic skills by operating within the abdominal cavity, an area more familiar to them given likely previous experience with laparoscopy, before progressing to the thorax. However, uptake of robotic surgery for neck pathology and the cervical esophagus is currently limited according to the published literature.18,114 This may explain the greater use of an open approach among the included studies.
In four studies, a stapled esophagogastric anastomosis was formed within the cervical wound to restore continuity of the digestive tract.42,44,46,115 In comparison, two studies reported a hand-sewn anastomosis—one formed using the robotic platform and one extracorporeal.42,115 The reported AL rate was higher among those who underwent three-stage esophagectomy (12.5%) compared with two-stage (8.10%). This fits with the reported literature, that the incidence of AL is lower in two-stage than three-stage esophagectomy, regardless of whether open, thoraco-laparoscopic or robotic procedures are performed.116
Strengths and Limitations
This is a comprehensive review of outcomes of robotic esophagectomy for resectable esophageal cancer, as evidence by the evaluation of over 100 studies that fulfilled the inclusion criteria. The range of key clinical parameters analysed cover the entirety of the patient’s hospital admission. Assessment of oncological outcomes scrutinises the potential benefits of robot-assisted esophageal resection further by taking into consideration the impact of radical surgery and lymphadenectomy on disease- and recurrence-free survival. Meta-analysis of clinical and oncological outcomes objectively validates the findings of the included studies; however, as demonstrated in Table 2, there was a significant degree of heterogeneity between the included studies in a number of outcomes. Uptake of robotic surgery is not consistent internationally and this is reflected in the reported outcomes. Furthermore, there were only four clinical trials on robotic esophageal cancer resection. These may limit the conclusions that can be drawn from current evidence. Data from prospective trials comparing open, thoraco-laparoscopic and hybrid procedures with robot-assisted esophagectomy are therefore required in order to make more definitive conclusions on the advantages of robotic surgery for resectable esophageal cancer.
Conclusions
This systematic review presents numerous advantages to perioperative outcomes conferred by robot-assisted surgery for resectable esophageal cancer. We have identified reduced intraoperative blood loss, shorter LoS, and greater LNY as being particularly advantageous. However, it is not yet clear that robotic surgery leads to a difference in survival in resectable esophageal cancer. This review highlights the presence of a learning curve and a minimum number of cases that may need to be performed before noticing marked improvement in postoperative outcomes afforded by robot-assisted surgery. Before standardised adoption of the robotic approach over current techniques, multicentre comparative clinical trials must be undertaken to identify the true objective benefit on perioperative and medium- and long-term outcomes. These may include involvement of longitudinal and circumferential resection margins, return to normal physical activities and work, and QOL, DFS and OS. The latter three should be benchmarked as standardised outcomes to determine whether the robotic platform affords an advantage in patient-reported and oncological outcomes.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg.. 2019;269(4):621–30.
Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet.. 2019;393(10184):1948–57.
van Hagen P, Hulshof MCCM, Lanschot JJB, et al. preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med.. 2012;366(22):2074–84.
Wang H, Tang H, Fang Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg.. 2021;156(5):444–51.
Mehdorn A-S, Möller T, Franke F, et al. Long-term, health-related quality of life after open and robot-assisted ivor-lewis procedures- a propensity score-matched study. J Clin Med.. 2020;9(11):3513.
Biere SSAY, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet.. 2012;379(9829):1887–92.
Paireder M, Asari R, Kristo I, et al. Morbidity in open versus minimally invasive hybrid esophagectomy (MIOMIE): long-term results of a randomized controlled clinical study. Eur Surg.. 2018;50(6):249–55.
Brierley RC, Gaunt D, Metcalfe C, et al. Laparoscopically assisted versus open oesophagectomy for patients with oesophageal cancer- the Randomised Oesophagectomy: Minimally Invasive or Open (ROMIO) study: protocol for a randomised controlled trial (RCT). BMJ Open.. 2019;9(11):e030907.
Nuytens F, Lenne X, Clément G, et al. Effect of phased implementation of totally minimally invasive ivor lewis esophagectomy for esophageal cancer after previous adoption of the hybrid minimally invasive technique: results from a french nationwide population-based cohort study. Ann Surg Oncol.. 2022;29(5):2791–801.
Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg.. 2003;238(4):486–94.
Briez N, Piessen G, Torres F, Triboulet JP, Mariette C. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br J Surg.. 2012;99(11):1547–53.
Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med.. 2019;380(2):152–62.
Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable oesophageal cancer: a meta-analysis. World J Surg Oncol.. 2016;14(1):304.
Washington K, Watkins JR, Jay J, Jeyarajah R. Oncologic resection in laparoscopic versus robotic transhiatal esophagectomy. JSLS.. 2019;23(2):e2019.00017.
van Boxel GI, Kingma BF, Voskens FJ, Ruurda JP, van Hillegersberg R. Robotic-assisted minimally invasive esophagectomy: past, present and future. J Thorac Dis.. 2020;12(2):54–62.
Ahmed K, Khan MS, Vats A, et al. Current status of robotic assisted pelvic surgery and future developments. Int J Surg.. 2009;7(5):431–40.
Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery Initial experience in foregut surgery. Surg Endosc.. 2002;16(12):1790–2.
Seto Y, Mori K, Aikou S. Robotic surgery for esophageal cancer: Merits and demerits. Ann Gastroenterol Surg.. 2017;1(3):193–8.
Grimminger PP, Staubitz JI, Perez D, et al. Multicenter experience in robot-assisted minimally invasive esophagectomy – a comparison of hybrid and totally robot-assisted techniques. J Gastrointest Surg.. 2021;25(10):2463–9.
Rahouma M, Baudo M, Mynard N, et al. Volume outcome relationship in post-esophagectomy leak: a systematic review and meta-analysis. Int J Surg Epub.. 2023. https://doi.org/10.1097/JS9.00000000000000420.
Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Community Medicine, University of Ottawa; 2020.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials.. 1996;17(1):1–12.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ.. 2009;339:b2535.
Hoelzen JP, Sander KJ, Sesia M, et al. Robotic-assisted esophagectomy leads to significant reduction in postoperative acute pain: a retrospective clinical trial. Ann Surg Oncol.. 2022;29(12):7498–509.
Egberts J-H, Welsch T, Merboth F, et al. Robotic-assisted minimally invasive Ivor Lewis esophagectomy within the prospective multicentre German da Vinci Xi registry trial. Langenbecks Arch Surg.. 2022;407(4):1–11.
Yang Y, Li B, Hua R, et al. Robot-assisted versus conventional minimally invasive esophagectomy for resectable esophageal squamous cell carcinoma: early results of a multicenter randomized controlled trial: the RAMIE trial. Ann Surg.. 2022;275(4):646–53.
Tagkalos E, van der Sluis PC, Berlth F, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer.. 2021;21(1):1060.
Chao Y-K, Wen Y-W, Chuang W-Y, Cerfolio R. Transition from video-assisted thoracoscopic to robotic esophagectomy: a single surgeon’s experience. Dis Esoph.. 2020;33(2):doz033.
Nickel F, Probst P, Fischer-Studier A, et al. Minimally Invasive Versus open AbdominoThoracic Esophagectomy for oesophageal carcinoma (MIVATE) – study protocol for a randomized controlled trial DRKS00016773. Trials.. 2021;22:41.
Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open.. 2020;3(1):e1918911.
Li H, Wu H, Cao M, et al. Long-term incidence rates of esophageal squamous cell carcinoma in chinese patients with low-grade intraepithelial neoplasia and association of surveillance endoscopy with incidence. JAMA Netw Open.. 2022;5(12):e2247415.
GBD 2017, Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its atributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol.. 2020;5(6):582–97.
van der Horst S, Weijs TJ, Ruurda JP, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum. J Thorac Dis.. 2017;9(Suppl 8):S834–42.
Angehern FV, Neuschütz KJ, Fourie L, et al. From open Ivor Lewis esophagectomy to a hybrid robotic-assisted thoracoscopic approach: a single-center experience over two decades. Langenbecks Arch Surg.. 2022;407(4):1421–30.
Jeong DM, Kim JA, Ahn HJ, Yang M, Heo BY, Lee SH. Decreased incidence of postoperative delirium in robot-assisted thoracoscopic esophagectomy compared with open transthoracic esophagectomy. Surg Laparosc Endosc Percutan Tech.. 2016;26(6):516–22.
Sayed AI, Goel S, Aggarwal A, Singh S. Robot assisted minimally invasive esohagectomy: safety, perioperative morbidity and short-term oncological outcome- a single institution experience. J Robot Surg.. 2022;16(3):517–25.
Goel A, Shah SH, Selvakumar VPP, Garg S, Kumar K. Robot-assisted mckeown esophagectomy is feasible after neoadjuvant chemoradiation our initial experience. Indian J Surg.. 2018;80(1):24–9.
Angehern FV, Neuschütz KJ, Fourie L, et al. Continously sutured versus linear-stapled anastomosis in robot-assisted hybrid Ivor Lewis esophageal surgery following neoadjuvant chemoradiotherapy: a single-center cohort study. Surg Endosc.. 2022;36(12):9435–43.
Park SY, Kim DJ, Kang DR, Haam SJ. Learning curve for robotic esophagectomy and dissection of bilateral recurrent laryngeal nerve nodes for esophageal cancer. Dis Esophagus.. 2017;30(12):1–9.
Kingma BF, Hadzijusufovic E, Van der Sluis PC, et al. A structured training pathway to implement robot-assisted minimally invasive esophagectomy: the learning curve results from a high-volume center. Dis Esophagus.. 2020;33(Suppl):2.
Han Y, Zhang Y, Zhang W, et al. Learning curve for robot-assisted Ivor Lewis esophagectomy. Dis Esophagus.. 2022;35(2):doab026.
Richter F, Mehdorn A-S, Fedders T, et al. C-reactive protein as predictor for infectious complications after robotic and open esophagectomies. J Clin Med.. 2022;11(19):5654.
Pointer DT Jr, Saeed S, Naffouje SA, et al. Outcomes of 350 robotic-assisted esophagectomies at a high-volume cancer center: a contemporary propensity-score matched analysis. Ann Surg.. 2022;276(1):111–8.
Palanivelu C, Dey S, Sabnis S, et al. Robotic-assisted minimally invasive oesophagectomy for cancer: an initial experience. J Minim Access Surg.. 2019;15(3):234–41.
Gong L, Jiang H, Yue J, et al. Comparison of the short-term outcomes of robot-assisted minimally invasive, video-assisted minimally invasive, and open esophagectomy. J Thorac Dis.. 2020;12(3):916–24.
Duan X, Gong L, Yue J, et al. Influence of induction therapy on robot-assisted mckeown esophagectomy for esophageal squamous cell carcinoma. Dig Surg.. 2020;37(6):463–71.
Wang F, Zhang H, Qiu G, Wang Z, Li Z, Wang Y. Double-docking technique, an optimized process for intrathoracic esophagogastrostomy in robot-assisted ivor lewis esophagectomy. Front Surg.. 2022;9:811836.
Kernstine KH, DeArmond DT, Shamoun DM, Campos JH. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc.. 2007;21(12):2285–92.
Peng H, Liu YY, Aimudula M, et al. A safe and effective anastomotic technique for robot-assisted minimally invasive oesophagectomy: Reverse-puncture anastomosis. Int J Med Robot.. 2022;18(1):e2336.
Egberts J-H, Stein H, Aselmann H, Hendricks A, Becker T. Fully robotic da Vinci Ivor-Lewis esophagectomy in four-arm technique – problems and solutions. Dis Esophagus.. 2017;30(12):1–9.
Voeten DM, van der Werf LR, Gisbertz SS, et al. Postoperative intensive care unit stay after minimally invasive esophagectomy shows large hospital variation. results from the dutch upper gastrointestinal cancer audit. Eur J Surg Oncol.. 2021;47(8):1961–8.
Meredith K, Blinn P, Maramara T, Takahashi C, Huston J, Shridhar R. Comparative outcomes of minimally invasive and robotic-assisted esophagectomy. Surg Endosc.. 2020;34:814–20.
Tsunoda S, Obama K, Hisamori S, et al. Lower incidence of postoperative pulmonary complications following robot-assisted minimally invasive esophagectomy for esophageal cancer: propensity score-matched comparison to conventional minimally invasive esophagectomy. Ann Surg Oncol.. 2021;28(2):639–47.
Defize IL, van der Horst S, Bülbul M, et al. Salvage robot-assisted minimally invasive esophagectomy (RAMIE) for T4b esophageal cancer after definitive chemoradiotherapy. Ann Surg Oncol.. 2021;28(5):2730–8.
van der Sluis PC, Ruurda JP, Verhage RJJ, et al. Oncologic long-term results of robot-assisted minimally invasive thoraco-laparoscopic esophagectomy with two-field lymphadenectomy for esophageal cancer. Ann Surg Oncol.. 2015;22(Suppl 3):S1350–6.
Sarkaria IS, Rizk NP, Grosser R, et al. Attaining proficiency in robotic-assisted minimally invasive esophagectomy while maximizing safety during procedure development. Innovations (Phila).. 2016;11(4):268–73.
Espinoza-Mercado F, Imai TA, Borgella JD, et al. Does the approach matter? Comparing survival in robotic, minimally invasive, and open esophagectomies. Ann Thorac Surg.. 2019;107(2):378–85.
Zhang Y, Han Y, Gan Q, et al. Early outcomes of robot-assisted versus thoracoscopic-assisted ivor lewis esophagectomy for esophageal cancer: a propensity score-matched study. Ann Surg Oncol.. 2019;26(5):1284–91.
de la Fuente SG, Weber J, Hoffe SE, Shridhar R, Karl R, Mereditch KL. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc.. 2013;27(9):3339–47.
Kamel MK, Sholi AN, Rahouma M, et al. National trends and perioperative outcomes of robotic oesophagectomy following induction chemoradiation therapy: a National Cancer Database propensity-matched analysis. Eur J Cardiothorac Surg.. 2020;59:ezaa336.
Naffouje SA, Salloum RH, Khalaf Z, Salti GI. Outcomes of open versus minimally invasive ivor-lewis esophagectomy for cancer: a propensity- score matched analysis of NSQIP database. Ann Surg Oncol.. 2019;26(7):2001–10.
Coker AM, Barajas-Gamboa JS, Cheverie J, et al. Outcomes of robotic-assisted transhiatal esophagectomy for esophageal cancer after neoadjuvant chemoradiation. J Laparoendosc Adv Surg Tech A.. 2014;24(2):89–94.
Cerfolio RJ, Wei B, Hawn MT, Minnich DJ. Robotic esophagectomy for cancer: early results and lessons learned. Sprig.. 2016;28(1):160–9.
Chouliaras K, Attwood K, Brady M, et al. Robotic versus thoraco-laparoscopic minimally invasive Ivor-Lewis esophagectomy, a matched-pair single-center cohort analysis. Dis Esophagus.. 2022;36(1):doac037.
Meredith K, Huston J, Andacoglu O, Shridhar R. Safety and feasibility of robotic-assisted Ivor-Lewis esophagectomy. Dis Esophagus.. 2018;31(7):doy005.
Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg.. 2013;145(1):90–6.
Somashekhar SP, Jaka RC. Total (transthoracic and transabdominal) robotic radical three-stage esophagectomy – initial indian experience. Indian J Surg.. 2017;79(5):412–7.
Keeney-Bonthrone TP, Abott KL, Haley C, et al. Transhiatal robot-assisted minimally invasive esophagectomy: unclear benefits compared to traditional transhiatal esophagectomy. J Robot Surg.. 2022;16:883–91.
Konstanidis IT, Ituarte P, Woo Y, et al. Trends and outcomes of robotic surgery for gastrointestinal (GI) cancers in the USA: maintaining perioperative and oncologic safety. Surg Endosc.. 2020;34(11):4932–42.
Chao Y-K, Tsai C-Y, Illias AM, Chen C-Y, Chiu C-H, Chuang W-Y. A standardized procedure for upper mediastinal lymph node dissection improves the safety and efficacy of robotic McKeown oesophagectomy. Int J Med Robot.. 2021;17(3):e2244.
Grimminger PP, Tagkalos E, Hadzijusufovic E, Corvinus F, Babic B, Lang H. Change from hybrid to fully minimally invasive and robotic esophagectomy is possible without compromises. Thorac Cardiovasc Surg.. 2019;67(7):589–96.
Yun JK, Chong BK, Kim HJ, et al. Comparative outcomes of robot-assisted minimally invasive versus open esophagectomy in patients with esophageal squamous cell carcinoma: a propensity score-weighted analysis. Dis Esophagus.. 2020;33(5):doz071.
Deng H-Y, Huang W-X, Li G, et al. Comparison of short-term outcomes between robot-assisted minimally invasive esophagectomy and video-assisted minimally invasive esophagectomy in treating middle thoracic esophageal cancer. Dis Esophagus.. 2018;31(8):doy012.
Chen J, Liu Q, Zhang X, et al. Comparisons of short-term outcomes between robot-assisted and thoraco-laparoscopic esophagectomy with extended two-field lymph node dissection for resectable thoracic esophageal squamous cell carcinoma. J Thorac Dis.. 2019;11(9):3874–80.
Deng H-Y, Luo J, Li S-X, et al. Does robot-assisted minimally invasive esophagectomy really have the advantage of lymphadenectomy over video-assisted minimally invasive esophagectomy in treating esophageal squamous cell carcinoma? A propensity score-matched analysis based on short-term outcomes. Dis Esophagus.. 2019;32(7):doy110.
Capovilla G, Hadzijusufovic E, Tagkalos E, et al. End to side circular stapled anastomosis during robotic-assisted Ivor Lewis minimally invasive esophagectomy (RAMIE). Dis Esophagus.. 2022;35(8):doab088.
Giulini L, Nasser CA, Tank J, Papp M, Stein HJ, Dubecz A. Hybrid robotic versus hybrid laparoscopic Ivor Lewis oesophagectomy: a case-matched analysis. Eur J Cardiothorac Surg.. 2021;59(6):1279–85.
Oshikiri T, Goto H, Horikawa M, et al. Incidence of recurrent laryngeal nerve palsy in robot-assisted versus conventional minimally invasive mckeown esophagectomy in prone position: a propensity score-matched study. Ann Surg Oncol.. 2021;28(12):7249–57.
Duan X, Yue J, Chen C, et al. Lymph node dissection around left recurrent laryngeal nerve: robot-assisted vs. video-assisted McKeown esophagectomy for esophageal squamous cell carcinoma. Surg Endosc.. 2021;35(11):6108–16.
Chao Y-K, Hsieh M-J, Liu Y-H, Liu H-P. Lymph node evaluation in robot-assisted versus video-assisted thoracoscopic esophagectomy for esophageal squamous cell carcinoma: a propensity-matched analysis. World J Surg.. 2018;42(2):590–8.
Betzler J, Elfinger L, Büttner S, et al. Robot-assisted esophagectomy may improve perioperative outcome in patients with esophageal cancer – a single-center experience. Front Oncol.. 2022;12:966321.
Park S, Hwang Y, Lee HJ, Park IK, Kim YT, Kang CH, et al. Comparison of robot-assisted esophagectomy and thoracoscopic esophagectomy in esophageal squamous cell carcinoma. J Thorac Dis.. 2016;8(10):2853–61.
Huang Y-H, Chen K-C, Lin S-H, Huang P-M, Yang P-W, Lee J-M. Robotic-assisted single-incision gastric mobilization for minimally invasive oesophagectomy for oesophageal cancer: preliminary results. Eur J Cardiothorac Surg.. 2020;58(Suppl 1):i65–9.
Kim D-J, Hyung WJ, Lee CY, et al. Thoracoscopic esophagectomy for esophageal cancer: feasibilitiy and safety of robotic assistance in the prone position. J Thorac Cardiovasc Surg.. 2010;139(1):53-59.e1010.
Osaka Y, Tachibana S, Ota Y, et al. Usefulness of robot-assisted thoracoscopic esophagectomy. Gen Thorac Cardiovasc Surg.. 2018;66(4):225–31.
Weijs TJ, van Eden HWJ, Ruurda JP, et al. Routine jejunostomy tube feeding following esophagectomy. J Thorac Dis.. 2017;9(Suppl 8):S851-860.
Rubinkiewicz M, Witowski J, Su M, Major P, Pedziwiatr M. Enhanced recovery after surgery (ERAS) programs for esophagectomy. J Thorac Dis.. 2019;11(Suppl 5):S685–91.
Weindelmayer J, Mengardo V, Gasparini A, et al. Enhanced recovery after surgery can improve patient outcomes and reduce hospital cost of gastrectomy for cancer in the west: a propensity-score-based analysis. Ann Surg Oncol.. 2021;28(12):7097–7094.
Babic B, Müller DT, Jung J-O, et al. Robot-assisted minimally invasive esophagectomy (RAMIE) vs. hybrid minimally invasive esophagectomy: propensity score matched short-term outcome analysis of a European high-volume center. Surg Endosc.. 2022;36(10):7747–55.
Charalabopoulos A, Davakis S, Syllaios A, Lorenzi B. Intrathoracic hand-sewn esophagogastric anastomosis in prone position during totally minimally invasive two-stage esophagectomy for esophageal cancer. Dis Esophagus.. 2021;34(6):doaa106.
Daiko H, Oguma J, Fujiwara H, et al. Robotic esophagectomy with total mediastinal lymphadenectomy using four robotic arms alone in esophageal and esophagogastric cancer (RETML-4): a prospective feasibility study. Esophagus.. 2021;18(2):203–10.
Morimoto Y, Kawakubo H, Ishikawa A, et al. Short-term outcomes of robot-assisted minimally invasive esophagectomy with extended lymphadenectomy for esophageal cancer compared with video-assisted minimally invasive esophagectomy: a single-center retrospective study. Asian J Endosc Surg.. 2022;15(2):270–8.
National Oesophago-Gastric Cancer Audit (NOGCA) Annual Report. 2022. Available at: https://www.nogca.org.uk/content/uploads/2023/01/REF378_NOGCA_2022-Annual-Report_FINAL-V1.1.pdf. Accessed 1 Aug 2022.
Jeremiasen M, Linder G, Hedberg J, et al. Improvements in esophageal and gastric cancer care in Sweden-population-based results 2007–2016 from a national quality register. Dis Esophagus.. 2020;33(3):doz070.
Sagar PM, Johnston D, McMahon MJ, Dixon MF, Quirke P. Significance of circumferential resection margin involvement after oesophagectomy for cancer. Br J Surg.. 1993;80(11):1386–8.
Pultrum BB, Honing J, Smit JH, et al. A critical appraisal of circumferential resection margins in esophageal carcinoma. Ann Surg Oncol.. 2010;17(3):812–20.
Depypere L, Moons J, Lerut T, et al. Prognostic value of the circumferential resection margin and its definitions in esophageal cancer patients after neoadjuvant chemoradiotherapy. Dis Esophagus.. 2018;31(2):dox117.
Sujendran V, Wheeler J, Baron R, Warren BF, Maynard N. Effect of neoadjuvant chemotherapy on circumferential margin positivity and its impact on prognosis in patients with resectable oesophageal cancer. Br J Surg.. 2008;95(2):191–4.
Schlick CJR, Khorfan R, Odell DD, Merkow RP, Bentrem DJ. Adequate lymphadenectomy as a quality measure in esophageal cancer: is there an association with treatment approach? Ann Surg Oncol.. 2020;27(11):4443–56.
Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw.. 2019;17(7):855–83.
Pucher PH, Green M, Bateman AC, et al. Variation in histopathological assessment and association with surgical quality indicators following oesophagectomy. Br J Surg.. 2021;108(1):74–9.
Ross SB, Rayman S, Thomas J, et al. Evaluating the cost for robotic vs “non-robotic” transhiatal esophagectomy. Am Surg.. 2022;88(3):389–93.
Galvani CA, Gorodner MV, Moser F, et al. Robotically assisted laparoscopic transhiatal esophagectomy. Surg Endosc.. 2008;22(1):188–95.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med.. 2006;355:11–20.
Lee Y, Samarasinghe Y, Lee MH, et al. Role of adjuvant therapy in esophageal cancer patients after neoadjuvant therapy and esophagectomy: a systematic review and meta-analysis. Ann Surg.. 2022;275(1):91–8.
Bott RK, Beckmann K, Zylstra J, et al. Adjuvant therapy following oesophagectomy for adenocarcinoma in patients with a positive resection margin. Br J Surg.. 2020;107(13):1801–10.
Dezube AR, Kucukak S, De León LE, Kostas K, Jaklitsch MT, Wee JO. Risk of chyle leak after robotic versus video-assisted thoracoscopic esophagectomy. Surg Endosc.. 2022;36(2):1332–8.
Guerra F, Gia E, Minuzzo A, Tribuzi A, Di Marino M, Coratti A. Robotic esophagectomy: results from a tertiary care Italian center. Updates Surg.. 2021;73(3):839–45.
Peri A, Furbetta N, Viganò J, Pugliese L, et al. Technical details for a robot-assisted hand-sewn esophago-gastric anastomosis during minimally invasive Ivor Lewis esophagectomy. Surg Endosc.. 2022;36(2):1675–82.
Wang F, Zhang H, Zheng Y, Wang Z, Geng Y, Wang Y. Intra-thoracic side-to-side esophagogastrostomy with a linear stapler and barbed suture in robot-assisted Ivor Lewis esophagectomy. J Surg Oncol.. 2019;120(7):1142–7.
Oesophago-Gastric Anastomosis Audit study group on behalf of the West Midlands Research Collaborative. The influence of anastomotic techniques on postoperative anastomotic complications: results of the oesophago-gastric anastomosis audit. J Thorac Cardiovasc Surg.. 2022;164(3):674-684.e5.
de Groot EM, Goense L, Kingma BF, van den Berg JW, Ruurda JP, van Hillegersberg R. Implementation of the robotic abdominal phase during robot-assisted minimally invasive esophagectomy (RAMIE): results from a high-volume center. Surg Endosc.. 2023;37(2):1357–65.
De Virgilio A, Constantino A, Festa BM, et al. Oncological outcomes of squamous cell carcinoma of the cervical esophagus treated with definitive (chemo-)radiotherapy: a systematic review and meta-analysis. J Cancer Res Clin Oncol.. 2023;149(3):1369–71.
Garas G, Tolley N. Robotics in otorhinolaryngology – head and neck surgery. Ann R Coll Surg Engl.. 2018;100(Suppl 7):34–41.
Xu Y, Li X-K, Cong Z-Z, et al. Long-term outcomes of robotic-assisted versus thoraco-laparoscopic McKeown esophagectomy for esophageal cancer: a propensity score-matched study. Dis Esophagus.. 2021;34(9):doaa114.
Xing H, Hu M, Wang Z, Jiang Y. Short-term outcomes of Ivor Lewis vs McKeown esophagectomy: a meta-analysis. Front Surg.. 2022;9:950108.
Acknowledgment
This systematic literature review represents independent research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The authors would like to thank The Royal Marsden Cancer Charity (Fund 83) for their support.
Funding
No financial or material support were received in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Nikhil Manish Patel, Pranav Harshad Patel, Kai Tai Derek Yeung, David Monk, Borzoueh Mohammadi, Muntzer Mughal, Ricky Harminder Bhogal, William Allum, Nima Abbassi-Ghadi, and Sacheen Kumar have no disclosures of any commercial interest in the subject of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patel, N.M., Patel, P.H., Yeung, K.T.D. et al. Is Robotic Surgery the Future for Resectable Esophageal Cancer?: A Systematic Literature Review of Oncological and Clinical Outcomes. Ann Surg Oncol 31, 4281–4297 (2024). https://doi.org/10.1245/s10434-024-15148-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15148-5