Abstract
Background
The morbidity and mortality rates of esophageal squamous cell carcinoma (ESCC) are high in China. The overall survival (OS) of patients with ESCC is related to lymph node (LN) metastasis (LNM). This study aimed to discuss the impact of metastasis in LN stations on the OS of patients with pathologic N1 (pN1) ESCC.
Methods
Data were obtained from the Esophageal Cancer Case Management database of Sichuan Cancer Hospital and Institute (SCCH-ECCM). Additionally, data of patients with pN1-category ESCC collected between January 2010 and December 2017 were retrospectively analyzed.
Results
Data from 807 patients were analyzed. The median OS of the patients with one metastatic LN (group 1) was 49.8 months (95 % confidence interval [CI], 30.8–68.9 months), whereas the OS of those with two metastatic LNs (group 2) was only 33.3 months (P = 0.0001). Moreover, group 1 did not show a significantly longer OS than group 2.1 (patients with 2 metastatic LNs in 1 LNM station; P = 0.5736), but did show a significantly longer OS than group 2.2 (patients with 2 metastatic LNs in 2 LNM stations; P < 0.0001). After propensity score-matching, the 5-year survival rate for group 1 was 28 %, whereas that for group 2 was 14 % (P = 0.0027).
Conclusions
The OS for the patients with one metastatic LN in one LNM was not significantly longer than for the patients with two metastatic LNs in one LNM station. Patients with one LNM station had a significantly longer OS than those with two LNM stations. Thus, the number of LNM stations is a significant determinant of OS in pN1 ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Global Cancer statistics have shown that esophageal squamous cell carcinoma (ESCC) has high morbidity and mortality rates, with higher occurrence rates among men than among women.1 Older age,2 smoking, and alcohol consumption are important risk factors.3,4,5 The treatment methods for ESCC depend on the tumor stage and location, histologic type, performance status, and comorbidities.
Surgery, radiotherapy, and chemotherapy are the standard treatment methods for ESCC.6,7,8,9,10 However, a diagnosis confirmed at an advanced stage of ESCC poses treatment challenges for many patients with ESCC.1 In addition, due to the delayed diagnosis and poor therapeutic effect of existing treatment methods, the 5-year overall survival (OS) rate is only approximately 30 %.1,2
Each of the currently available treatment methods works differently. For example, radiotherapy uses high-energy electron beams to shrink the tumor and destroy it.11 In contrast, chemotherapy involves using drugs as infusions or pills to reduce tumor size and inhibit the tumor’s growth.12 Immunotherapy activates the immune system to recognize and destroy tumor cells.13
With advances in technology and research, gene therapy, tumor vaccines, and other new techniques are being developed to treat esophageal cancer.14,15 However, surgery through radical tumor resection continues to be the cornerstone of comprehensive treatment for ESCC.6,7,9,16 Lymph node (LN) metastasis (LNM) poses a significant challenge to the curative effect of the standard treatment options.17,18,19,20
Currently, the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) tumor-node-metastasis (TNM) staging and the Japan Esophageal Society (JES) system are the internationally used criteria for cancer staging systems.9,21 According to the UICC/AJCC TNM staging, pathologic N1 (pN1) is defined as one or two metastatic LNs. However, as per the 11th Japanese Classification of Esophageal Cancer, based on tumor location, different LN stations are divided into groups: N1, N2, and N3.9,21 The two systems differ in terms of the LN (N) category. Unlike UICC/AJCC, the JES system focuses more on metastasis of the LN location.9,21 The main purpose of this retrospective study was to discuss the impact of metastasis in different LN stations on the OS of patients with pN1-category ESCC.
Methods
Study Design and Patients
Data from 2957 patients with ESCC treated at Sichuan Cancer Hospital (SCCH) from January 2010 to December 2017 were retrospectively reviewed. Data and medical records of the patients were obtained from the database of the hospital’s Esophageal Cancer Case Management (SCCH-ECCM database). The data extracted included demographics (sex and age), tumor factors such as pathologic disease stage (T and N categories), TNM stage, location and grade, clinical disease stage (lymphovascular invasion, nerve invasion, LNM, metastasis in LN station), and clinical treatment method (radical resection).
At the discretion of individual surgeons and based on patient characteristics, 797 patients underwent right transthoracic esophagectomies with two- or three-field LN dissection. Overall, 579 patients underwent the Mckeown method, 218 patients underwent the Ivor-Lewis method, and 669 patients underwent intraoperative thoracic duct ligation. The clinical staging of each patient was discussed by experts before treatment and surgery. After surgery, the surgeon separated the specimens to obtain the LNs, which were named according to the guidelines. The final pathologic results were interpreted by two pathologists and re-signed by another pathologist. The disease stage was classified according to the UICC/AJCC eighth-edition TNM staging system.
Regarding adjuvant treatment, 127 (15.7 %) patients received postoperative adjuvant chemoradiotherapy (CRT), 308 (38.0 %) patients received postoperative adjuvant chemotherapy (CT), and 17 (2.1 %) patients received postoperative radiotherapy (RT). Hereafter, we refer to this collectively as surgery plus postoperative CT or RT/CRT. The specific types of drugs and their doses were determined based on individual patient characteristics and discussed by experts before treatment. Details of the clinical treatment methods are shown in Table S1.
The inclusion criteria for the analysis specified (1) patients who underwent esophagectomy in our hospital and (2) patients classified as pN1. The exclusion criteria ruled out patients who had (1) pathology-confirmed non-squamous cell carcinoma, (2) extra-thoracic tumor location, (3) preoperative neoadjuvant therapy, (4) distant metastasis of tumor, and (5) missing data (Fig. 1).
Patients were followed up once every 3 months for the first 2 years, then once every 6 months thereafter for 3 to 5 years. Overall survival (OS) was defined from the month and year of surgery to death or last follow‑up visit in April 2022.
All procedures performed in this study were in accordance with the Declaration of Helsinki as revised in 2013. Due to the retrospective nature of the study, the Ethics Committee waived consent from patients.
Criterion of Adverse Events and Characteristic
The patients were divided into two groups: group 1 (patients with 1 metastatic LN according to pathologic results) and group 2 (patients with 2 metastatic LNs). The patients in group 2 were further divided into two subgroups: group 2.1 (patients with metastasis in 1 LN station) and group 2.2 (patients with metastasis in 2 LN stations).
Statistical Analysis
Categorical variables are presented as percentages. Results were calculated using the chi-square test or Fisher’s exact test. The OS rate was calculated based on the time from the month and year of surgery to the date of death or last follow-up evaluation. Hazard ratios (HRs) and 95 % confidence intervals (CIs) were calculated. Independent OS risk factors were identified using univariate Cox regression analyses. Cox proportional hazards regression models were used to assess the impact of all baseline covariates on outcomes. Kaplan–Meier curves were created using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) to evaluate the observation system. Log-rank tests were used to describe the median at specific time points in 95 % CI.
Furthermore, two comparable groups of patients (groups 1 and 2; groups 1 and 2.1; or groups 1 and 2.2) were created using propensity score-matching (PSM) and adjusting unbalanced covariates. For PSM, the caliper was set to a width of 0.02, and the 1:1 closest-match method was used. The PSM procedure analyzed all the variables in the baseline profile (sex, age, pathologic differentiation grade, lymphovascular invasion, nerve invasion, tumor location, pathologic T category, eighth-edition TNM stage, thoracic surgery, abdominal surgery, and clinical treatment method).
A P value lower than 0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 23.0 (SPSS, Chicago, IL, USA).
Results
Clinical Outcomes
For the analysis, 807 patients with pN1-category ESCC were selected. Group 1 included 504 (62.5 %) patients with one metastatic LN, and group 2 included 303 (37.5 %) patients with two metastatic LNs. Group 2.1 included 94 (11.6 %) patients, and group 2.2 included 209 (25.9 %) patients.
In terms of age, 765 (94.8 %) of the patients were younger than 75 years, and 42 (5.2 %) were 75 years of age or older. Furthermore, 662 (82.0 %) of the patients were males, and 145 (18.0 %) were females. Stage III or IV disease was recorded for 754 (93.4 %) of the patients (Table 1). Statistical differences were observed between groups 1 and 2 in terms of lymphovascular invasion (P = 0.028) and eight TNM stages (P = 0.02). After PSM, the two groups were comparable.
Survival
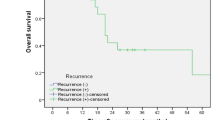
After a median follow-up time of 65.6 months, the median OS of 807 patients was 41.9 months (95 % CI 36.8–47.0 months). The median OS of the patients in group 1 was 49.8 months (95 % CI 30.8–68.9 months), whereas the OS of the patients in group 2 was 33.3 months (95 % CI 28.3–38.3 months). The 1-, 3-, and 5-year OS rates were 88 %, 52 %, and 28 %, respectively, for the group 1 patients, whereas these values were 87 %, 40 %, and 14 %, respectively, for the group 2 patients (HR, 0.67; 95 % CI 0.55–0.81; P = 0.0001; Fig. 2A). Subgroup analyses showed no statistically significant difference in OS between groups 1 and 2.1 (HR, 0.88; 95 % CI 0.65–1.18; P = 0.5736; Fig. 2C). However, the OS was significantly longer for the patients in group 1 than for those in group 2.2 (HR, 0.61; 95 % CI 0.49–0.75; P < 0.0001; Fig. 2E). Because postoperative adjuvant therapy has a significant impact on OS, relevant subgroup analyses were further performed (Fig. 3). Details of postoperative adjuvant therapy are shown in Tables S1 and S2.
A Overall survival curve of patients with metastasis in one lymph node (LN) and patients with metastasis in two LNs before propensity score-matching. B Overall survival curve of patients with one LN metastasis and patients with two LN metastases after score-matching. C Overall survival curve of patients with one LN metastasis and patients with two LN metastases in one LN station before propensity score-matching. D Overall survival curve of patients with one LN metastasis and patients with two LN metastases in one LN station after propensity score-matching. E Overall survival curve of patients with one LN metastasis and patients with two LN metastases in two LN stations before propensity score-matching. F Overall survival curve of patients with one LN metastasis and patients with two LN metastases in two LN stations after propensity score-matching
A Overall survival curve of analyzed adjuvant therapy patients with one lymph node (LN) metastasis and patients with two LN metastases. B Overall survival curve of analyzed adjuvant therapy patients with one LN metastasis and patients with two LN metastases in one LN station. C Overall survival curve of analyzed adjuvant therapy patients with one LN metastasis and patients with two LN metastases in two LN stations. D Overall survival curve of analyzed patients between surgery alone and postoperative therapy
A significant difference in the OS was observed between the patients in groups 1 and 2 after PSM analyses. The 3- and 5-year survival rates for the patients in group 1 were 50 % and 28 %, respectively, whereas those for the patients in group 2 were 40 % and 14 %, respectively (HR, 0.76; 95 % CI 0.61–0.94; P = 0.0027; Fig. 2B). In comparison, the 3- and 5-year OS rates for the patients in group 2.1 were 53 % and 20 %, respectively (HR, 0.52; 95 % CI 0.34–0.78; P = 0.2258; Fig. 3D). No significant difference in OS was observed between groups 1 and 2.1 after PSM. However, a significant difference in the OS was observed between the patients in groups 1 and 2.2 after PSM analyses (HR, 0.72; 95 % CI 0.56–0.93; P = 0.0037; Fig. 3F).
Factors significantly affecting OS after esophagectomy were identified using univariate analyses. These factors included sex (P < 0.001), age (P = 0.016), tumor grade (P = 0.075), lymphovascular invasion (P = 0.002), nerve invasion (P = 0.03), pathologic T category (P < 0.001), TNM stage according to the UICC/AJCC eighth edition (P = 0.009), clinical treatment method (P = 0.01), number of metastatic LNs (P = 0.003), and number of metastatic LN stations (P < 0.001; Table 2). Multivariate analyses showed that sex (P < 0.001), age (P = 0.007), tumor grade (P = 0.016), lymphovascular invasion (P = 0.026), pathologic T category (P < 0.001), and clinical treatment method (P = 0.001) were crucial factors affecting 5-year OS after esophagectomy (Table 2).
Discussion
Medical records in the SCCH-ECCM database of the patients who underwent esophagectomy for ESCC were retrospectively analyzed. Based on the grouping of the patients and the pathologic status, our results indicated that the patients with one metastatic LN had significantly longer OS than those with two sites (P = 0.0001). However, the OS for the patients with one metastatic LN station was not significantly longer than for the patients with two metastatic LNs in one metastatic LN station (P = 0.5736). Moreover, the patients with one metastatic LN had a significantly longer OS than the patients with two metastatic LNs in two LN stations (P < 0.0001). These findings remained after PSM, showing no significant difference in the OS between the patients in groups 1 and 2 (P = 0.0027) and no significant difference between the patients in groups 1 and 2.1 (P = 0.2258). However, a significant difference was observed in the OS between the patients in groups 1 and 2.2 (P = 0.0037). In addition, the number of metastatic LNs and the stations of metastatic LNs exhibited significant effects (P = 0.003 vs. P < 0.001) after multivariate analyses. We believe that metastasis in LN stations is one of the crucial predictors affecting OS after esophagectomy. Thus, we can infer that for patients with pN1 ESCC, the number of metastatic LN stations can lower the OS compared with metastatic LNs.
The current standard treatment method for ESCC includes primarily comprehensive treatment through surgery.7,9,16,21 Patients may receive chemotherapy, chemoradiotherapy, or immunotherapy combined with chemoradiotherapy before esophagectomy.7,16,22,23 After esophagectomy, additional immunotherapy is considered.24 However, the extent of lymphadenectomy in esophagectomy still is a topic of continued discussion.18,19,20,25,26 Considering the pattern of metastasis in ESCC, LN stations have certain clinical value and are gaining increased importance.8,27
The TNM staging criteria of the JES system are being progressively developed and enhanced, and the transition from the 11th to the 12th Japanese Classification of Esophageal Cancer highlights the shift from the location-based classification to the number-based classification akin to UICC/AJCC TNM staging.9 However, TNM staging using the AJCC/UICC system emphasizes the number of resected metastatic LNs and LN stations.21 The data in this study focused not only on the LNM number but also on the LNM stations. It was based on the pN1 status identified using the AJCC/UICC staging system. Although the OS for the patients with one metastatic LN was higher than for those with two metastatic LNs, if two metastatic LNs were located in the different LN stations, this lowered the OS. However, the OS did not show a statistically significant difference in the patients with two metastatic LNs in one metastatic LN station. This further suggests a scope for improvement in the staging system.
Several studies have confirmed that different LN stations have distinct clinical values.8,27,28 Ma et al.28 showed that the resection of subcarinal and left tracheobronchial LNs has a high clinical value.28,29 Furthermore, subcarinal LNM has been shown to decrease the OS of patients. According to the JES system, subcarinal LNM was classified as the pN2 category for upper, middle, and lower thoracic ESCC.9 Another example is the left tracheobronchial LN station. Although LN resection of this station can improve OS, because of the high difficulty level associated with the procedure and the potential risk of serious complications, even life-threatening effects resulting from improper management, the necessity of lymphadenectomy in all stations is debatable.29 Thus, the factors of LNs that affect OS are complex and multi-sourced. Nevertheless, relevant studies and our research currently confirm that systematic LN dissection is necessary to accurately diagnose the pathological stage of LNs and improve the prognosis of survival in patients.
Our study had limitations. First, although numerous studies on the pattern of LN metastasis have been conducted, the precise pattern of LN metastasis remains uncertain. The importance of each LN station in OS still is controversial and heterogeneous. Second, although the size of the samples from high-volume thoracic surgery centers was relatively large, this was a single-center retrospective analysis. Third, due to heterogeneity, after PSM, the sample size for statistical analysis decreased by hundreds of cases. Future studies using data from multicenter prospective clinical trials are warranted for further assessment of the LN stations, clinical value, and TNM staging.
Conclusions
The OS for the patients with one LNM was not significantly longer than for the patients with two LNs in one metastatic LN station. Moreover, patients with one metastatic LN station had a significantly longer OS than those with two LN stations. Thus, the number of metastatic LN stations is a significant determinant of OS for patients with pN1-category ESCC.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
National Cancer Institute. SEER Cancer Stat Facts: Esophageal Cancer. Retrieved 20 January 2021 at https://seer.cancer.gov/statfacts/html/esoph.html.
Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580–93.
Rumgay H, Shield K, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. 2021;22:1071–80.
La Vecchia C, Liati P, Decarli A, et al. Tar yields of cigarettes and the risk of oesophageal cancer. Int J Cancer. 1986;38:381–5.
Obermannova R, Alsina M, Cervantes A, et al. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment, and follow-up. Ann Oncol. 2022;33:992–1004.
Shapiro J, van Lanschot J, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8.
Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus. 2016;13:1–7.
Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th edition: part I. Esophagus. 2017;14:1–36.
Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, Version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:855–83.
Park S, Oh D, Choi YL, et al. Durvalumab and tremelimumab with definitive chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancer. 2022;128:2148–58.
Faried A, Faried LS, Kimura H, et al. Differential sensitivity of paclitaxel-induced apoptosis in human esophageal squamous cell carcinoma cell lines. Cancer Chemother Pharmacol. 2006;57:301–8.
Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms, and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15.
Hsieh CH, Kuan WH, Chang WL, et al. Dysregulation of SOX17/NRF2 axis confers chemoradiotherapy resistance and emerges as a novel therapeutic target in esophageal squamous cell carcinoma. J Biomed Sci. 2022;29:90.
Guo YJ, Che XY, Shen F, et al. Effective tumor vaccines generated by in vitro modification of tumor cells with cytokines and bispecific monoclonal antibodies. Nat Med. 1997;3:451–5.
Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36:2796–803.
Abe S, Tachibana M, Shiraishi M, et al. Lymph node metastasis in resectable esophageal cancer. J Thorac Cardiovasc Surg. 1990;100:287–91.
Li K, Leng X, He W, et al. Resected lymph nodes and survival of patients with esophageal squamous cell carcinoma: an observational study. Int J Surg. 2023;10.1097.
Li K, Nie X, Li C, et al. Mapping of lymph node metastasis and efficacy index in thoracic esophageal squamous cell carcinoma: a large-scale retrospective analysis. Ann Surg Oncol. 2023;10.1245.
Li K, Du K, Liu K, et al. Impact of two-field or three-field lymphadenectomy on overall survival in middle and lower thoracic esophageal squamous cell carcinoma: a single-center retrospective analysis. Oncol Lett. 2023;25:189.
Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th edn. New York: Springer; 2016.
Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (Palace-1). Eur J Cancer. 2021;232–41.
Kato K, Ito Y, Daiko I, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG 1109 NExT study. J Clin Oncol. 2022;40:238.
Patel MA, Kratz JD, Lubner SJ, Loconte NK, Uboha NV. Esophagogastric cancers: integrating immunotherapy therapy into current practice. J Clin Oncol. 2022;24:2751–62.
Fujita H, Kakegawa T, Yamana H et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg. 1995;5:654–62.
Li B, Zhang Y, Miao L, et al. Esophagectomy with three-field versus two-field lymphadenectomy for middle and lower thoracic esophageal cancer: long-term outcomes of a randomized clinical trial. J Thorac Oncol. 2021;2:310–7.
Li K, Leng XF, Du K, et al. Clinical value and efficacy index of lymphadenectomy for thoracic esophageal squamous cell carcinoma. Dis Esophagus. 2022; 2(Suppl):362.
Ma H, Li Y, Ding Z, Liu X, Xu J, Qin J. The clinical significance of subcarinal lymph node dissection in the radical resection of oesophageal cancer. Interact Cardiovasc Thorac Surg. 2013;16:839–43.
Xu L, Wei XF, Chen XK, et al. Clinical significance of left tracheobronchial lymph node dissection in thoracic esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2022;164:1210–9.
Acknowledgement
This work was supported by grants from the National Key Research and Development Program (Grant No. 2022YFC2403400), the International Cooperation Projects of the Science and Technology Department of Sichuan Province (Grant No. 24GJHZ0166), the Sichuan Key Research and Development Project from the Science and Technology Department of Sichuan Province (Grant No. 2022YFQ0008, 2023YFS0044, 2023YFQ0056, 2023YFS0488, and 2023YFQ0055), the Wu Jieping Clinical Research Projects (Grant Nos. 320.6750.2020-15-3), and the Sichuan Province Clinical Key Specialty Construction Project. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors: Study concept and design; acquisition, analysis, or interpretation of data; critical revision of the article for important intellectual content; and final approval of the version to be published. Kexun Li: Drafting of the article. Kunyi Du: Revision of the article. Changding Li and Xin Nie: Statistical analysis. Lin Peng: Obtaining funding. Yongtao Han and Lin Peng: Administrative, technical, and material support. Lin Peng and Xuefeng Leng: Study supervision. Kexun Li, Kunyi Du, and Changding Li had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Disclosures
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Partial preliminary results of this article were presented as a poster by the first author at the 18th World Congress for Esophageal Diseases was held in Tokyo, Japan, from September 26 to 28, 2022.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, K., Du, K., Li, C. et al. Impact of Metastatic Lymph Nodes on Survival of Patients with pN1-Category Esophageal Squamous Cell Carcinoma: A Long-Term Survival Analysis. Ann Surg Oncol 31, 3794–3802 (2024). https://doi.org/10.1245/s10434-024-15019-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15019-z