Abstract
Background
Systemic and local recurrences of urothelial bladder cancer (UBC) significantly impair survival after radical cystectomy (RC), but little is known about the impact of the recurrence of urothelial cancer in the upper urinary tract (UTUC). This report describes survival outcomes and their predictors for patients who underwent RC followed by radical nephroureterectomy (RNU) for UTUC.
Methods
The Surveillance, Epidemiology, and End Results database was queried to identify patients who underwent RC for UBC and subsequent RNU for UTUC. The Kaplan–Meier method and competing-risk Cox regression (CRR) were used for the survival analysis.
Results
Overall, 102 patients have undergone RNU within a median of 49 months (interquartile range [IQR], 27–76 months) since RC. Muscle-invasive UTUCs were predominant at RNU (n = 58; 56.7%), but organ-confined bladder tumors were most frequent at RC (n = 42, 41.5%). After RNU, the estimated 5-year overall survival (OS) was 25.9%, the cancer-specific survival (CSS) was 35.6%, the median OS was 23 months (IQR, 11–63 months), and the CSS was 34 months (IQR, 13–132 months). In the multivariable CRR, the factors predictive for CSS after RNU included male gender (hazard ratio [HR], 2.36; 95% confidence interval [CI], 1.03–5.42; p < 0.05), muscle-invasive UTUC (HR, 2.20; 95% CI, 1.13–4.28; p < 0.05), and the presence of distant metastasis (HR,11.59; 95% CI, 5.33–25.2; p < 0.001).
Conclusions
In conclusion, the patients who underwent RNU for UTUC after RC for UBC experienced poor OS and CSS. The majority of RNUs were performed for locally advanced tumors. The independent risk factors for worse OS and CSS after RNU were UTUC T stage, presence of metastasis, and male gender.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bladder cancer constitutes a major risk factor for the development of upper urinary tract urothelial cancer (UTUC).1 Radical cystectomy (RC) remains a mainstay surgical treatment for muscle-invasive bladder cancer (MIBC) and for selected patients with high-risk non-muscle-invasive bladder cancer (NMIBC).2,3 Obviously, patients who undergo RC are at greater risk for UTUC than the general population.4,5
The estimated frequency of UTUC in RC cohorts is about 3%, although it is reported to range from 0.75% to 6.4% in different studies.5,6 In such patients, radical nephroureterectomy (RNU) due to urothelial cancer (UC) recurrence in the upper urinary tract (UUT) should be strongly considered, particularly for high-risk tumors. However, RNU after RC, might be especially challenging considering the burden of comorbidities and anatomic abnormalities contributing to the increased complexity of surgery. Due to the high competing mortality, RNU seems to be rarely chosen for UTUC management of these patients.4 A review of contemporary UTUCs in the post-RC follow-up evaluation reported that only 61% of such patients underwent RNU.5
Information about survival outcomes of RNU after RC is derived from small retrospective cohorts.7,8 One of these cohorts, consisting of 34 patients, reported a 5-year cancer-specific survival (CSS) of 45.6%, and another cohort with 64 patients showed a median overall survival (OS) of 3.1 years after RNU.7,8
The reported incidence of UTUC after RC seems to be lower than expected considering the field-effect theory of urothelial carcinogenesis and lower than that reported for high-risk NMIBC treated with Bacillus Calmette-Guerin (BCG) therapy (from 7.5% to even 25% within a 15-year follow-up period).9,10 Such discrepancy might be attributable to the high competing risk of other events, leading to a substantial mortality burden after RC. Importantly, early morbidity after RC due to gastrointestinal, infectious, and cardiovascular complications is relatively high, and the perioperative mortality rate is about 3%.11,12,13
Rapid bladder cancer recurrence and progression are other significant factors contributing to increased early mortality after RC.12 The majority of metastatic progressions after RC occur within 2 years after surgery, especially in the case of locally advanced bladder tumors.4 On the other hand, long-term survivors after RC represent a group with a substantial risk for metachronous UTUC.14,15
In this study, we aimed to report survival outcomes of RNU after RC and identify their predictors.
Methods
The National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database was queried to identify patients with bladder cancer (International Classification of Diseases [ICD]-10 codes C67.0–C67.9) and subsequent UTUC (ICD-10 codes C65.9–C66.9) treated surgically between 2004 and 2019. We included patients who underwent RC for bladder cancer (procedure codes 50–80) and subsequent RNU for primary metachronous UTUC (procedure codes 40–50) using the “site-specific surgery of primary-site code” variable. Exceptionally, to assess the impact of RNU on survival among cystectomized patients, we included an additional risk-matched cohort of RC patients solely for survival comparison using the Kaplan–Meier method.
The available data included information on the patient’s demographics as well as histopathologic and clinical characteristics of bladder cancer and UTUC from the time of RC and RNU, respectively. Generally, survival was calculated from the time of RNU. Cancer-specific survival was defined as the interval between UTUC diagnosis and death due to UC. Overall survival was defined as the interval between UTUC diagnosis and death due to any cause. Exceptionally, in the comparison of bladder cancer patients treated with RC between those who did and those who did not experience UTUC (treated with RNU), we calculated the survival time starting from the bladder cancer diagnosis (Fig. 1).
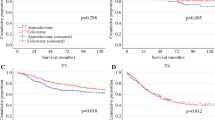
Comparison of a cancer-specific survival and b overall survival between propensity score-matched patients who underwent RNU for metachronous UTUC (red curve) after RC and their counterparts treated with RC alone (blue curve). Kaplan–Meier curves depict conditional cancer-specific survival from the landmark points of c 12 and e 36 months since RC performance. Kaplan–Meier curves depict conditional overall survival from the landmark points of d 12 and f 36 months since RC performance. RNU, radical nephroureterectomy; UTUC, urinary tract urothelial cancer; RC, radical cystectomy
Ethics
The institutional review board waived the need for study approval. The study was performed in accordance with the Declaration of Helsinki and its later amendments.
Statistical Analysis
The Kaplan–Meier method and log-rank tests were used in survival analyses. The median OS and CSS, as well as survival rates, were estimated using the Kaplan–Meier method. Kaplan Meier curves with 95% confidence intervals (CIs) were used to illustrate survival. Conditional survival was analyzed using a landmark approach based on Kaplan–Meier estimates and calculated for patients alive 12 and 36 months after cystectomy (Fig. 1C–F). Cox proportional hazards (CPHs) were used for the prediction of survival outcomes in uni- and multivariable analyses. Competing risk regression (CRR) with CPH was performed to identify the risk factors for CSS. Stepwise selection of variables was used for multivariable analyses. Only statistically significant variables entered the final multivariable model. Hazard ratio (HR) supplemented with a 95% CI were derived from CPH and CRR. Two-sided p values lower than 0.05 were considered as statistically significant. Statistical analyses were performed with SAS software version 9.4.
Subgroup Analysis to Evaluate the Impact of RNU on Survival of RC Patients
To examine the effect of RNU on survival among cystectomized patients, propensity score (PS)-matching was performed to adjust for confounders such as patient’s age, gender, race, bladder cancer T stage and grade, bladder cancer N and M stages at RC, lymph node dissection (LND) during RC, use of perioperative chemotherapy, and year of bladder cancer diagnosis. Bladder cancer patients who received RNU for UTUC after RC were PS-matched in the proportion of 1:2 with those who did not.
Results
The study included 102 patients who underwent RC with subsequent RNU. The median follow-up period after RC was 91 months (interquartile range [IQR], 50–158 months). The majority of the patients were males (n = 82, 80.4%) who were younger than 70 years at the time of their RC (n = 59, 57.8%). Of the 102 patients, 92 were white (90.2%), only 4 (3.9%) were black, and 6 (5.9%) were of other races.
Stages of Bladder Cancer and UTUC at the Radical Surgery
At the time of RC, 42 patients (41.2%) had a diagnosis of NMIBC, and 41 patients (40.2%) had a diagnosis of MIBC. There was a strong predominance of high-grade urothelial bladder cancer (n = 75, 73.5%). Lymph node (LN) dissection during RC was performed for 88 patients (87.1%), and positive LNs were diagnosed in 6 patients (5.9%). The types of urinary diversion included an ileal conduit in 32 patients (31.4%), an orthotopic neobladder in 1 patient (1%), an abdominal pouch in 1 patient (1%), and a nonspecified type in the remaining 70 patients (66.6%).
Importantly, at RNU, the muscle-invasive stages of UTUC (MIUC) (n = 58, 56.7%) were significantly more common than the non-muscle-invasive stages of UTUC (NMIUC) (n = 34, 33.3%). Of the 102 patients, 70 (68.3%) had high-grade UTUCs and 32 (31.7%) had low-grade UTUCs. The UC originated from the renal pelvicalyceal system in 68 patients (67%) and from the ureter in 34 patients (33%). During RNU, LND was performed in 49 patients (48.5%). The findings showed 63 patients (61.8%) who were LN-negative, and 23 patients (22.6%) who were LN-positive (N1–N2). Distant metastases were diagnosed in 3 patients (2.9%) at the time of RC and 6 patients (5.9%) at the time of RNU. All baseline characteristics of the patients at the time of RC and RNU were presented in Table 1.
RNU Timing and Survival Outcomes
Due to the UTUC diagnosis, RNU was performed within a median of 49 months (IQR, 27–76 months) after RC. In the majority of the patients, UTUC was developed late after RC, and in only 37 individuals (36.3%) UTUC occured within 3 years after RC. Early perioperative 3-month all-cause mortality after RNU was 10.4% (n = 10).
Estimated survivals after RNU included a 2-year OS of 50.0%, a 5-year OS of 25.9%, a 2-year CSS of 56.2%, and a 5-year CSS of 35.6%. After RNU, the median OS was 23 months (IQR, 11–63 months), and the CSS was 34 months (IQR, 13–132 months).
Univariable Analyses for CSS and OS After RNU
In univariable competing-risk Cox regression analyses, the following factors were associated with CSS: age of 70 years or older at RC (HR, 1.95; 95% CI, 1.16–3.27; p < 0.05), age of 70 years or older at RNU (HR, 1.67; 95% CI, 0.98–2.86; p = 0.06), male gender (HR, 2.51; 95% CI, 1.01–6.20; p < 0.05), race (other vs. white: HR, 2.53; 95% CI, 1.15–5.58; p < 0.05), UTUC T stage (MIUC vs. NMIUC: HR, 2.78; 95% CI, 1.46–5.31; p < 0.01), UTUC N stage (N1-2 vs. N0: HR, 2.42; 95% CI, 1.27–4.60; p < 0.01), and metastasis at RNU (M1 vs. M0: HR, 12.76; 95% CI, 5.83–27.96; p < 0.001) (Table 2).
In univariable Cox regression analyses, the following factors were associated with OS: age of 70 years or older at RC (HR, 2.07; 95% CI, 1.28–3.34; p < 0.01), age of 70 years or older at RNU (HR, 2.20; 95% CI, 1.35–3.58; p < 0.01), male gender (HR, 2.25; 95% CI, 1.04–4.90; p < 0.05), metastasis at RC (M1 vs. M0: HR, 4.38; 95% CI, 1.82–10.58; p = 0.001); UTUC T stage (MIUC vs. NMIUC: HR, 2.85; 95% CI, 1.66–4.90; p < 0.001), UTUC N stage (N1-2 vs. N0: HR, 1.82; 95% CI, 1.00–3.31; p = 0.05), metastasis at RNU (M1 vs. M0: HR, 8.62; 95% CI, 4.11–18.08; p < 0.0001), and interval between bladder cancer and UTUC diagnosis (<3 vs. >3 years: HR, 1.56; 95% CI, 0.99–2.46; p = 0.056) (Table 3).
Multivariable Analysis for CSS and OS After RNU
In the multivariable competing-risk Cox regression analysis, the factors predictive for cancer-specific mortality after RNU were male gender (HR, 2.36; 95% CI, 1.03–5.42; p < 0.05), stage at RNU (MIUC vs. NMIUC: HR, 2.20; 95% CI, 1.13–4.28; p < 0.05), and presence of distant metastasis at RNU (M1 vs. M0: HR, 11.59; 95% CI, 5.33–25.2; p < 0.001) (Table 2).
In the multivariable Cox regression analysis, the factors predictive for overall mortality after RNU were age older than 70 years at RNU (HR, 2.21; 95% CI, 1.25–3.92; p < 0.01), gender (male vs. female: HR, 2.64; 95% CI, 1.14–6.11; p < 0.05) disease stage at RNU (MIUC vs. NMIUC: HR, 3.29; 95% CI, 1.73–6.27; p < 0.001), presence of distant metastasis at RNU (M1 vs. M0: HR, 7.88; 95% CI, 2.92–21.25; p < 0.001), presence of distant metastasis at RC (M1 vs. M0: HR, 9.33; 95% CI, 1.91–45.5; p < 0.001), and interval between bladder cancer and UTUC diagnosis (<3 vs. >3 years: HR, 2.71; 95% CI, 1.51–4.86; p < 0.001) (Table 3).
Survival Outcomes After RC with Subsequent RNU Compared with RC Alone
To evaluate whether UTUC recurrence impairs survival outcomes after RC, CSS (Fig. 1A) and OS (Fig. 1B) were compared between risk-stratified, PS-matched patients who underwent RNU for metachronous UTUC after RC and their counterparts treated with RC alone. In the Kaplan–Meier analyses of PS-matched cohorts, UTUC requiring RNU after RC compared with no RNU was initially not associated with worse OS (p = 0.81) but with worse CSS (p = 0.02). Additional conditional survival analyses from the landmark point of 12 and 36 months after RC, showed a worse CSS (both p < 0.001) for the patients who had developed UTUC. A negative impact of UTUC on OS was observed in the 36-month landmark analysis (p = 0.02).
Discussion
This study analyzed the survival outcomes and their predictors in patients who underwent RNU for metachronous UTUC after previous RC for bladder cancer. First, we observed very poor survival after RNU due to metachronous UTUC recurrence in patients previously treated with RC. Half of the patients succumbed within 2 years after RNU, with the majority of deaths attributable to cancer-specific causes. Second, although the majority of the patients had organ-confined bladder cancer at the time of RC, they presented with muscle-invasive UTUC at the time of RNU. Third, most often, RNU for UTUC recurrence was a late event after RC. The interval between surgeries exceeded 3 years for the majority of the patients. Furthermore, RNU was associated with a non-negligible, relatively high 3-month all-cause mortality rate of 10%. Fourth, in the PS-matched, risk-stratified cohort of bladder cancer patients treated with RC, RNU for UTUC was associated with worse CSS, and a landmark analysis at 3 years time point, showed a detrimental effect on OS. Fifth, the independent risk factors associated with cancer-specific mortality after RNU were distant metastases at RNU, UTUC T stage, and male gender.
Pan-urothelial cancer with metachronous recurrence in the UUT after RC constitutes an aggressive disease with unsatisfactory treatment outcomes.4 In our observation, almost two thirds of patients following RNU after RC succumb to urothelial cancer within 5 years, and 3 of 4 patients die from all causes within 5 years. Therefore, radical surgical treatment does not provide satisfactory cancer control for UTUC in that setting, and our findings align with previous studies with smaller sample sizes.7,8 Poor survival after RNU in patients previously treated with RC had been reported in only a few retrospective studies with small cohorts.4,7,8
We observed a relatively high frequency of UTUCs with adverse pathologic features at the time of RNU, with a predominance of pT3-pT4 stage disease (48% of patients) and a substantial proportion of lymph node-positive disease (22.6%). Additionally, we noted an underutilization of LND (only 49%) and perioperative chemotherapy (19.6%) at the time of RNU. High rates of locally advanced UTUCs after RC might be the consequence of suboptimal guideline adherence regarding intensity of imaging follow-up evaluation, use of non-contrast imaging due to impaired renal function with suboptimal sensitivity for UTUC detection, unsatisfactory accuracy of urine cytology, and finally, relatively late occurrence of UTUC after RC when intensity of surveillance has already been deescalated.
Perhaps all the aforementioned diagnostic methods seem not to provide a satisfactory sensitivity for metachronous UC detection.4,6 Other studies also have included a significant percentage of locally advanced UTUCs (pT3-pT4) and LN-positive disease at diagnosis.5,7 Notably, for cystectomized patients, UTUC is most commonly diagnosed due to symptoms including hematuria, flank pain, and pyelonephritis, which are already associated with advanced overlooked disease.5 A meta-analysis reported that 64% of patients with UC recurrence in the UUT experienced symptoms.6 These underscore the limitations of current follow-up schedules. Consequently, the majority of RNUs in our study were performed due to muscle-invasive and locally advanced UTUC, indicating the overlooked early-stage cancer.
On the other hand, the detection of asymptomatic disease is associated with a 30% reduction of mortality compared with symptomatic disease.16 The window of opportunity for effective treatment with RNU is missed when UTUC is diagnosed at a locally advanced stage, which strongly impairs the survival.17
As we have shown, the independent risk factors for worse CSS were muscle-invasive stage of UTUC, presence of distant metastasis at RNU, and male gender. These factors support a surveillance strategy for long-term bladder cancer survivors aimed at early detection of UC recurrence in remnant urothelium.
Currently, oncologic surveillance after RC involves regular chest and abdominopelvic computed tomography scans to detect local or distant recurrence of UC.2 No consensus exists concerning the regular performance of urine cytology, including urethral washing or urethroscopy for the purpose of early detection of metachronous UC recurrence.2,6
Overall survival was worse for the patients with muscle-invasive UTUC or metastasis at the time of RC or RNU, for males, for patient’s older than 70 years, and for patients with an interval between RC and RNU shorter than 3 years. These indicate that early diagnosis of UTUC at the time of the localized non-muscle-invasive stage increases the chances for better survival. On the other hand, age, male gender, and UTUC development early after RC constitute non-modifiable risk factors compromising survival.
A meta-analysis by Picozzi et al.6 indicated that the risk of UTUC after RC ranges from 0.75% to 6.4%. Especially at high-risk for recurrence in remnant urothelium were patients with multifocal NMIBC, including carcinoma in situ, bladder neck tumor, involvement of the prostatic urethra, and positive urothelial margins.4
Interestingly, in the current study, the patients with NMIBC at RC were overrepresented in the cohort of metachronous UTUCs due to the high competing mortality observed in the MIBC cohort. Stage T1 bladder cancer at the time of radical cystectomy was the most common in our study, likely due to the extended survival of these patients, allowing the development of UTUC.18 Notably, the indications for RC in the NMIBC setting include BCG-unresponsive disease, tumors not amenable to complete transurethral resection, and selected cases of high- and very-high-risk tumors, which are at significant risk of cancer-specific mortality.19
Failure of BCG therapy is relatively common, reported for approximately 20% of contemporary cohorts at high risk for NMIBC.20,21 Meta-analysis showed an almost threefold higher risk of UTUC after RC in superficial versus invasive bladder cancer.6 We identified a relatively low number of patients with locally advanced bladder cancer and subsequent RNU for UTUC, which might be attributable to very high competing short-term bladder cancer-specific mortality in these individuals. Perhaps even in the case of early UTUC recurrence for such patients, RNU is not routinely performed due to synchronous metastatic progression of bladder cancer. On the other hand, a significant proportion of patients with locally advanced bladder cancer at RC die due to bladder cancer recurrence and metastasis and do not live long enough for UTUC to develop.
Another important finding of our study was the late recurrence of metachronous UTUC after RC. The majority of UTUCs occur 3 years after RC, and within that period, the significant percentage of cystectomized patients with pT3-pT4 bladder cancer experience progression and decease.4,5 In our study, the median interval between RC and RNU was 49 months, which seems to be consistent with a meta-analysis reporting a median of 43 months between RC and UTUC recurrence.5
The reported poor survival following RNU after RC underscores the need for improvement. Administration of adjuvant chemotherapy and performance of LND as an integral part of RNU have been shown to prolong survival in UTUC, but both were underused in the studied cohort.22,23,24 Notably, the timing of the UTUC diagnosis seemed to have the greatest impact on survival, highlighting the importance of ongoing surveillance for long-term RC survivors.
In the initial analysis of the PS-matched cohort of RC patients, those with subsequent RNU for UTUC had worse CSS but not OS compared with the individuals without UTUC requiring RNU. An additional landmark analysis with 12- and 36-month time points demonstrated a worse CSS for the patients who experienced the development of metachronous UTUC.
Analysis of conditional survival among patients alive 36 months after RC showed that further development of UTUC resulted in worse OS. These results confirm the detrimental effect of UTUC on both CSS and OS. Performing RNU after previous RC can be a technically challenging surgery that requires a high level of surgical skills due to the potential complications related to urinary diversion and the post-RC anatomic and functional changes of the urinary tract. The difficulty of RNU might be dependent on the type of urinary diversion and the UTUC stage.
The perioperative all-cause mortality reported in our study was high, exceeding 10% within 3 months after surgery. This is a significantly higher rate than that observed in the primary RNU setting. Our results can help clinicians in counseling long-term RC survivors at risk of late recurrence in the UUT.
The limitations of our study included its retrospective character and missing baseline data for some patients, as reported in Table 1. On the other hand, regarding the uniqueness of the cohort, the population-based character of the study enabled us to gather a sufficient number of patients for the analysis. Selection bias was inevitable due to the inclusion of patients who survived bladder cancer and lived long enough to experience the development of metachronous UTUC. In unrestricted survival analysis, the lack of difference in OS between those who received RNU after RC and those who underwent RC alone might be the result of selection bias. Simultaneously, it underscores the high prevalence of competing mortality causes in patients after RC. However, conditional survival with a 36-month landmark point showed a worse OS for the patients who experienced the development of UTUC during the subsequent follow-up evaluation.
It is noteworthy that in our cohort, perioperative chemotherapy for UTUC did not influence survival, but the absence of data on the regimen and number of cycles constituted a major confounder. The low utilization of chemotherapy in this cohort may have been attributable to the high morbidity burden and impaired renal function in a significant proportion of the patients. The absence of data regarding the status of surgical margins may be considered a limitation in the risk factor analysis.
Another limitation of our study was the lack of available data regarding the curative intent of the surgery for the analyzed patients. It is important to note that some of the patients may have undergone RNU due to symptoms or as a palliative procedure. The inclusion of a proportion of patients with metastatic UTUC (5.8% M1 and 4.9% Mx) and the observation of poor median overall survival after RNU suggest that at least some of the surgeries may not have been performed with a curative intent. This limitation raises the possibility that our findings could have been influenced by non-curative surgeries, emphasizing the need to interpret the results with caution in light of this uncertainty. On the other hand, it is worth noting that long-term survivors also were identified, with 25% of the cohort surviving more than 5 years afterward, which is evidence for disease cure. Reliable survival data and relatively long-term follow-up evaluation must be considered as strengths of the study.
Conclusion
In conclusion, patients who underwent RNU for UTUC after RC for bladder cancer experienced poor overall and cancer-specific survival. Metachronous UTUC recurrence is a late oncologic event after RC, placing long-term RC survivors at risk. The majority of RNUs are performed for muscle-invasive UTUC, which constitutes an adverse prognostic factor.
Data Availability
The datasets analyzed during the current study are available from the U.S. National Cancer Institute upon request.
References
van Doeveren T, van de Werken HJG, van Riet J, Aben KKH, van Leeuwen PJ, Zwarthoff EC, et al. Synchronous and metachronous urothelial carcinoma of the upper urinary tract and the bladder: are they clonally related? A systematic review. Urol Oncol Semin Orig Investig. 2020;38:590–8.
Professionals SO. Uroweb [cited 16 June 2021]. EAU guidelines: muscle-invasive and metastatic bladder cancer. Retrieved at https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/
Ślusarczyk A, Zapała P, Zapała Ł, Piecha T, Radziszewski P. Prediction of BCG responses in non-muscle-invasive bladder cancer in the era of novel immunotherapeutics. Int Urol Nephrol. 2019;51:1089–99.
Gakis G, Black PC, Bochner BH, Boorjian SA, Stenzl A, Thalmann GN, et al. Systematic review on the fate of the remnant urothelium after radical cystectomy. Eur Urol. 2017;71:545–57.
Sanderson KM, Rouprêt M. Upper urinary tract tumour after radical cystectomy for transitional cell carcinoma of the bladder: an update on the risk factors, surveillance regimens and treatments. BJU Int. 2007;100:11–6.
Picozzi S, Ricci C, Gaeta M, Ratti D, Macchi A, Casellato S, et al. Upper urinary tract recurrence following radical cystectomy for bladder cancer: a meta-analysis on 13,185 patients. J Urol. 2012;188:2046–54.
Li Q, Assel M, Benfante N, Pietzak E, Bagrodia A, Cha E, et al. Clinical outcomes in patients with panurothelial carcinoma treated with radical nephroureterectomy following cystectomy for metachronous recurrence. J Urol. 2017;198:546–51.
Aumatell J, Huguet J, Subiela JD, Gaya JM, Faba ÓR, Territo A, et al. Endoscopic exploration directly impacts clinical decision-making in the management of patients with suspected upper tract urothelial carcinoma following radical cystectomy. Urol Oncol. 2021;39:732.e1-e8.
Herr HW. Extravesical tumor relapse in patients with superficial bladder tumors. J Clin Oncol Off J Am Soc Clin Oncol. 1998;16:1099–102.
Nishiyama N, Hotta H, Takahashi A, Yanase M, Itoh N, Tachiki H, et al. Upper tract urothelial carcinoma following intravesical bacillus Calmette–Guérin therapy for nonmuscle-invasive bladder cancer: results from a multi-institutional retrospective study. Urol Oncol Semin Orig Investig. 2018;36:306.e9-e15.
Bochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol. 2015;67:1042–50.
Zapała Ł, Ślusarczyk A, Korczak B, Kurzyna P, Leki M, Lipiński P, et al. The view outside of the box: reporting outcomes following radical cystectomy using Pentafecta from a multicenter retrospective analysis. Front Oncol. 2022;12:841852.
Hladun T, Ratajczak J, Salagierski M. Can we lower the rates of cystectomy complications by modifying risk factors? A review of the literature. Cent Eur J Urol. 2022;75:28–34.
Miest T, Khanna A, Sharma V, Hensley PJ, Campbell R, Thapa P, et al. Development and validation of a risk-adapted scoring model for metachronous upper tract urothelial carcinoma following radical cystectomy. J Urol. 2022;207:284–92.
Miest TS, Sharma V, Boeri L, Tollefson MK, Thompson RH, Boorjian SA, et al. Does ureteral stent drainage prior to cystectomy increase the risk of subsequent upper tract urothelial carcinoma and ureteral complications? Urology. 2021;153:215–20.
Stewart-Merrill SB, Alahdab F, Benkhadra K, Wang Z, Sorita A, Boorjian SA, et al. Oncologic surveillance in bladder cancer following radical cystectomy: a systematic review and meta-analysis. Urol Oncol. 2016;34(236):e13-21.
Correia J, Mendes G, Texeira B, Madanelo M, Fraga A, Silva-Ramos M. Perioperative and oncological outcomes of laparoscopic and open radical nephroureterectomy for locally advanced upper tract urothelial carcinoma: a single-center cohort study. Cent Eur J Urol. 2022;75:257–64.
Ślusarczyk A, Zapała P, Olszewska-Ślusarczyk Z, Radziszewski P. The prediction of cancer-specific mortality in T1 non-muscle-invasive bladder cancer: comparison of logistic regression and artificial neural network: a SEER population-based study. Int Urol Nephrol. 2023;55:2205–13. https://doi.org/10.1007/s11255-023-03655-5.
Ślusarczyk A, Zapała P, Zapała Ł, Borkowski T, Radziszewski P. Cancer-specific survival of patients with non-muscle-invasive bladder cancer: a population-based analysis. Ann Surg Oncol. 2023;30(12):7892–902.
Ferro M, Barone B, Crocetto F, Lucarelli G, Busetto GM, Del Giudice F, et al. Predictive random-pathological factors to identify BCG, unresponsive patients, after re-resection for T1 high grade non-muscle invasive bladder cancer. Urol Oncol Semin Orig Investig. 2022;40:490.e13-e20.
Ślusarczyk A, Piotr Z, Garbas K, Zapała Ł, Borkowski T, Radziszewski P. Blood count-derived inflammatory markers predict time to Bacillus Calmette–Guérin failure in high-risk non-muscle-invasive bladder cancer. Arch Med Sci. 2021. https://doi.org/10.5114/aoms/130303.
Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomized controlled trial. Lancet. 2020;395:1268–77.
Ślusarczyk A, Zapała P, Piecha T, Rajwa P, Moschini M, Radziszewski P. The association between lymph node dissection and survival in lymph node-negative upper urinary tract urothelial cancer. Cancers. 2023;15:4660.
Roscigno M, Shariat SF, Margulis V, Karakiewicz P, Remzi M, Kikuchi E, et al. Impact of lymph node dissection on cancer-specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol. 2009;181:2482–9.
Funding
The authors declare that no funds, grants, or other financial support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AŚ contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by AŚ. The first draft of the manuscript was written by AŚ and all authors (PZ, TP, ŁZ, TB, PR) edited and commented on previous versions of the manuscript. The project was supervised by PR. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosure
There are no conflict of interest.
Ethical Approval
Due to the character of the study, no Ethics Committee approval was required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ślusarczyk, A., Zapała, P., Piecha, T. et al. Upper Urinary Tract Urothelial Cancer After Radical Cystectomy for Bladder Cancer: Survival Outcomes After Radical Nephroureterectomy. Ann Surg Oncol 31, 2144–2153 (2024). https://doi.org/10.1245/s10434-023-14710-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14710-x