Abstract
Background
Physical prehabilitation is recommended before major abdominal surgery to ameliorate short-term outcomes.
Methods
A frequentist, random-effects network meta-analysis (NMA) was performed to clarify which type of preoperative physical activity among aerobic exercise (AE), inspiratory muscle training (IMT), and resistance training produces benefits in patients who underwent major abdominal surgery. The surface under the P-score, odds ratio (OR), or mean difference (MD) with a 95% confidence interval (CI) were reported. The results were adjusted by using the component network approach. The critical endpoints were overall and major morbidity rate and mortality rate. The important but not critical endpoints were the length of stay (LOS) and pneumonia.
Results
The meta-analysis included 25 studies. The best approaches for overall morbidity rate were AE and AE + IMT (OR = 0.61, p-score = 0.76, and OR = 0.66, p-score = 0.68). The best approaches for pneumonia were AE + IMT and AE (OR = 0.21, p-score = 0.91, and OR = 0.52, p-score = 0.68). The component analysis confirmed that the best incremental OR (0.30; 95% CI 0.12–0.74) could be obtained using AE + IMT. The best approach for LOS was AE alone (MD − 1.63 days; 95% CI − 3.43 to 0.18). The best combination of components was AE + IMT (MD − 1.70; 95% CI − 2.06 to − 1.27).
Conclusions
Physical prehabilitation reduces the overall morbidity rate, pneumonia, and length of stay. The most relevant effect of prehabilitation requires the simultaneous use of AE and IMT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The major surgical procedures produce a significant homeostatic disturbance characterized by catabolism, increased oxygen demand, and inflammatory status.1 The stress response is associated with an increased risk of developing postoperative complications.2 In recent years, enhanced recovery after surgery (ERAS) has been proposed to reduce the risk of postoperative morbidity. One of the basic concepts of the ERAS approach was that the preoperative period could be used to optimize the overall conditioning of patients.3 The preoperative streamlining is called “prehabilitation” and includes correcting modifiable risk factors, such as anemia, malnutrition, smoking, and comorbidities.4,5 A not very well-known critical issue of prehabilitation is physical activity. For the patient, facing a major surgery can be compared with “running a marathon.” Thus, similar to a “marathon,” it requires specific and dedicated physical training. Although physical prehabilitation is yet recommended by ERAS guidelines,4,6,7 the approach used is not well defined, including a combination of three different techniques: aerobic exercises (AE), such as cycling and walking; resistance training (RT); and inspiratory muscle training (IMT). A recent meta-analysis clearly stated that the best combination for adequate physical prehabilitation is unknown.8 Our study was designed to fill this gap by overcoming the multiarm and multicomponent setting problem using a component network meta-analysis (CNMA).9 The CINeMA10 and GRADE11 approaches were used to present the results in an accessible form.
Material and Methods
The study protocol was preregistered in PROSPERO (CRD42023387987). The manuscript was structured following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (PRISMA).12
Eligibility Criteria
The eligibility criteria were based on the “Population-Intervention-Control-Outcomes-Studies” (PICOS) approach.13 The “Population” was represented by patients who underwent major abdominal surgery, excluding cholecystectomy, abdominal hernia repair, or obesity surgery. The “Intervention” arms considered any physical prehabilitation based on a hospital program. The “Control” group was called nonspecific training (NST) and included any preoperative approach without specific physical prehabilitation. When home activity is recommended without particular exercise, the arm was included the NST group. The “Studies” included only when they reported the morbidity, mortality, or length of stay and only if the design was randomized. CNMA was used to overcome the multiarm problem and isolate the weight of each component.
Information Source, Search, Study Selection, and Data Collection Process
The research was based on a previous classical meta-analysis,8 updating the systematic review. The last search was performed on January 23, 2023. The PubMed/Medline, Embase, and Cochrane databases were used. The search string was managed by using the SR accelerator14 and is reported in the Supplementary file.
Data Items
For each study, we described the first author, year of publication, affiliation, procedures, design (blinded or not), type of disease (malignant or benign), postoperative ERAS management, preoperative nutritional intervention, and compliance rate. The relevance of endpoints was judged by the panel of authors using the GRADE approach (not important, important, critical).15 The postoperative morbidity (overall and major) and mortality were considered “critical.” Major morbidity was defined according to the Clavien-Dindo class >2.16 The LOS, pneumonia, and postoperative 6-min walking test (6MWT)17 were considered “important.” The panel established the minimally important differences as follows18: for morbidity (overall and major), mortality, and pneumonia, 10 per 1000 persons more or fewer; for length of stay (LOS), at least ± one day; for 6MWT ± 50 meters. The studies were clustered in different arms based on the AE, RT, and IMT combination.
Geometries of the Network and Risk of Bias Within the Individual Study
The network geometry was plotted by using nodes and edges. The nodes were the different combinations of interventions, whereas the lines display the observed comparisons: the thickness of the lines is proportional to the number of studies. The frequency component combination was represented by using a dedicated plot.19 The risk of bias was based on the revised tool for assessing the risk of bias in randomized trials (Rob2).20 The indirectness was considered not negligible when the study population, interventions, and outcomes measurement were not entirely representative of PICOS criteria. Indirectness reduces the transitivity across the common nodes in NMA, returning challenging to obtain credible network estimates. The studies were evaluated as “low-risk,” “some concerns,” or “major concerns.” LA and FS performed Rob2 and indirectness evaluation.
Summary Measurements and Methods of the Analysis
Frequentist NMA by using random effect was performed. The effect estimates were measured as odds ratios (ORs) or mean differences (MDs), with 95% confidence intervals (CI). The results also were reported as p-score, which was the probability, without uncertainty, that combinations would be the best based on the outcome analyzed.21,22 The variety of interventions was considered among the best if p-score was ≥0.66; when p-score was 0.65 to 0.33, the combination was judged inferior to the best/better than the worst; when p-score was <0.33, the combination was considered among the worst. The effect of each component intervention also is estimated by CNMA, summing the relative impact of the components comprising this intervention.9 The effects of each component were reported as incremental OR (iOR) or MD (iMD) with CI. Results were tabulated according to the GRADE recommendation.23 All analysis was made by using the netmeta and viscomp package for R version 4.0.1.
Inconsistency, Risk of Bias Across the Studies, and Meta-Regression Analysis
The global and local incoherence were evaluated.24 The local incoherence was related to the unreliability of the networks, and it was described as the ratio of odds ratio (RoR) or difference of MD between direct and indirect estimates. The local incoherence was considered not negligible when the p-value was <0.05. The heterogeneity was measured with I2.25 Publication/reporting bias was investigated using the Begg test.26
Assessment of the Certainty of the Evidence
Based on the GRADE methodology,27 four levels of evidence were considered: (1) high quality, which means that the true effect lies close to that of the NMA estimates; (2) moderate quality, which means the actual effect is likely to be close to the NMA estimates, but there is a possibility that it is substantially different; (3) low quality, namely that the true effect may be substantially different from the NMA estimates; (4) shallow quality, which means the true effect is likely to be substantially different from the NMA estimates. The certainty of the evidence was obtained by using online CINeMA software by evaluating the following criteria: within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence.28 If some or major concerns are observed, the certainty is downgraded.
Results
Studies Selection, Characteristics, and Risk of Bias Within Studies
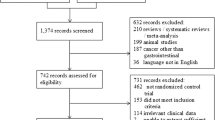
The systematic literature search following the PRISMA statement is reported in Supplementary Fig. 1. In Table 1, the characteristics of the 25 included studies are reported.29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53 The details about the prehabilitation programs of included studies are reported in Supplementary Table 1. The patients were clustered into the following intervention arms: Arm NST, including patients preoperative managed without specific training; Arm IMT + AE, including patients who received inspiratory muscle and resistance training; Arm AE + RT, including patients who received aerobic exercise and resistance training; Arm IMT, including patients who received only inspiratory muscle training; Arm AE, including patients who received only aerobic exercise; Arm RT, including patients who received only resistance training; Arm IMT + AE + RT, including patients who received simultaneously aerobic exercise, inspiratory muscle, and resistance training. The meta-analytic compliance results are shown in Fig. 1.
Network Structures and Geometries and Synthesis of Results
Overall Morbidity
A total of 2404 patients are available for this endpoint. The network geometry is reported in Fig. 2A, whereas the frequency of components is in Fig. 2B. Table 2 shows the results of classical NMA for the morbidity rate. In contrast, Figs. 2C–D show the funnel and forest plots, respectively. Heterogeneity was 50.3%, and global inconsistency was 0.323. Publication bias was absent (Begg test, p = 0.960). The assessment of evidence certainty and risk of bias were exhaustively reported in Supplementary Table 2. AE alone (p-score = 0.76) and AE + IMT (p-score = 0.68) can be considered among the best approaches, reducing the morbidity rate by nearly 1.5 times (OR = 0.61 and OR = 0.66, respectively). The estimated effect was 186 events fewer per 1000 patients treated for AE and 163 events fewer per 1000 patients treated combining AE + IMT. The certainty of the evidence was very low for AE alone for imprecision (95 CI OR include null effect line), significant heterogeneity (p = 0.009), and incoherence (conflict results among direct and indirect evidence). The certainty of the evidence was low for AE + IMT due to imprecision (95 CI OR include null effect line) and incoherence (conflict results among direct and indirect evidence), respectively.
Overall morbidity rate: Network geometry (A), Heat plot (B), Funnel plot (C), Forest plot (D), and Density plot (E). AE aerobic exercise; RT resistance training; IMT inspiratory muscle training; NST no specific training; OR odds ratio; p-score: the intervention is considered among the best if p-score was ≥0.66; when p-score was 0.65–0.33, the combination was judged inferior to the best/better than the worst; when p-score was <0.33, the combination was considered among the worst
The component analysis (Fig. 2E) showed that none of the components have significant incremental effect: AE, iOR = 0.61 (95% CI 0.35–1.07), p = 0.090; RT, iOR = 1.31 (95% CI 0.69–2.47), p = 0.404; IMT, iOR = 1.18 (95% CI 0.60–2.30), p = 0.902. Moreover, none of the combinations has a statistically significant incremental effect.
Major Overall Morbidity (CDC > 2)
The morbidity, according to the CDC, is extractable only from 1312 patients. The network geometry is reported in Supplementary Fig. 2A, whereas the frequency of components is in Supplementary Fig. 2B. None of the intervention arms was superior to NST (Supplementary Table 3 and Supplementary Fig. 2C–D). Heterogeneity (0%, p = 0.423), global inconsistency (0.001), and publication bias (Begg test, p = 0.790) were absent. The detail about the certainty of the evidence is in Supplementary Table 4. The component analysis showed that none of the components or possible combinations has a significant incremental effect. The density plot was not created due to the small number of studies.
Mortality
A total of 2674 patients are available for this endpoint. The network geometry is reported in Supplementary Fig. 3A, and the frequency of components is in Supplementary Fig. 3B. Supplementary Table 5 shows the results of classical NMA for the morbidity rate. In contrast, Supplementary Figs. 3C and D showed the forest and funnel plots, respectively. Heterogeneity (0%; p = 0.986), global inconsistency (tau2 = 0), and publication bias (Begg test, p = 0.920) were absent. The evidence certainty and risk of bias were exhaustively reported in Supplementary Table 6. None approach significantly reduced the mortality rate. The component analysis showed that none of each component or the possible combinations have a significant incremental effect, as reported in the density plot (Supplementary Fig. 3E).
Length of Stay
A total of 2468 patients are available for this endpoint. The network geometry is reported in Fig. 3A, whereas the frequency of components is shown in Fig. 3B. Table 3 shows the results of classical NMA. Figure 3C and D show the funnel and forest plots, respectively. Heterogeneity was 51.8%, and global inconsistency was 1.86. Publication bias was absent (Begg test, p = 0.600). The evidence certainty and risk of bias were reported in Supplementary Table 7. The only intervention arm among the best approaches was AE alone (p-score = 0.75). Patients treated with AE have a length of stay (LOS) of nearly 2 days inferior to those not treated (MD = − 1.63). The certainty of the evidence was very low for imprecision (95 CI OR include null effect line), significant heterogeneity (p = 0.010), and incoherence (conflict results among direct and indirect evidence).
Length of stay: Network geometry (A), Heat plot (B), Funnel plot (C), Forest plot (D), and Density plot (E). AE aerobic exercise; RT resistance training; IMT inspiratory muscle training; NST no specific training; MD mean difference; p-score: the intervention is considered among the best if p-score was ≥0.66; when p-score was 0.65–0.33, the combination was judged inferior to the best/better than the worst; when p-score was <0.33, the combination was considered among the worst
The component analysis showed that no component alone had a significant incremental effect: AE, iMD = − 0.99 (95% CI − 2.08 to 0.09), p = 0.075; IMT, iMD = − 0.41 (95% CI − 1.51 to 0.68), p = 0.460; RT, iOR = 0.56 (95% CI − 0.79 to 1.93), p = 0.415. On the contrary, AE + IMT was the only combination that guaranteed a significant reduction of LOS of nearly 2 days (iMD = − 1.7 days; −2.06 to − 1.27 CI; p < 0.001), as shown in Fig. 3E.
Pneumonia
A total of 1452 patients are available for this endpoint. The network geometry is reported in Fig. 4A, whereas the frequency of components is shown in Fig. 4B. Table 4 shows the results of classical NMA for the pneumonia rate. In contrast, Fig. 4C and D show the funnel and forest plots, respectively. Heterogeneity was 34.8% (p = 0.139), and global inconsistency was 0.431. Local inconsistency was tested due to the absence of closed loops. Publication bias was absent (Begg test, p = 0.220). The assessment of evidence certainty and risk of bias were exhaustively reported in Supplementary Table 8. AE + IMT alone (p-score = 0.91) and AE (p-score = 0.68) can be considered among the best approaches. AE + IMT reduced the risk of pneumonia (OR = 0.21), whereas AE (OR = 0.52) about half. The expected pneumonia rate was 138 fewer per 1000 patients when AE + IMT was administrated during the preoperative period. The expected pneumonia rate was 84 fewer per 1000 patients when AE was administrated in the preoperative period. The certainty of the evidence was low for AE + IMT due to imprecision (95 CI OR include null effect line) and incoherence (conflict results among direct and indirect evidence). The certainty of the evidence was very low for AE due to imprecision (95 CI OR include null effect line), significant heterogeneity (p = 0.010) and incoherence (conflict results among direct and indirect evidence). The component analysis showed that none of the components had a significant incremental effect: AE, iOR = 0.45 (95% CI 0.19–1.06), p = 0.066; IMT, iOR = 0.67 (95% CI 0.40–1.11), p = 0.119; RT, iOR = 3.02 (95% CI 0.97–9.41), p = 0.056.
Pneumoniae rate: Network geometry (A), Heat plot (B), Funnel plot (C), and Forest plot (D). AE aerobic exercise; RT resistance training; IMT inspiratory muscle training; NST no specific training; OR odds ratio; p-score: the intervention is considered among the best if p-score was ≥0.66; when p-score was 0.65–0.33, the combination was judged inferior to the best/better than the worst; when p-score < 0.33, the combination was considered among the worst
The calculated effect showed that AE + IMT was the only combination with a statistically significant effect. Combining AE + IMT, the pneumonia rate can be significantly reduced of three times: iOR of 0.30 (95% CI 0.12–0.74; p = 0.014).
6MWT
CNMA was not performed because, in 21 studies, this datum was not extractable or not reported.
Discussion
The present study attempts to clarify the role of physical prehabilitation before major abdominal surgery, specifying the weight of each exercise. The study included 25 randomized, controlled trials and 2674 patients, resulting in the largest meta-analysis. The main problem was that the interventional arms were a combination of three different types of supervised physical activity: AE, such as cycling and walking; RT, using elastic bands for different muscles; and IMT device-assisted. This problem was solved by using the NMA approach, while the CNMA methodology was used to weigh each component’s relevance and obtain the plausible best combinations.9 Moreover, CINeMA10,28 and GRADE11 approaches were used to overcome the simple evaluation of statistical significance. The quality of the evidence was evaluated, not only considering the imprecision (namely the statistical significance of effect size) but also testing within-study bias, reporting bias, indirectness, heterogeneity, and incoherence. The first exciting finding derived from the descriptive analysis: the meta-analytic compliance rate was not so low (88%), as suggested by several authors.41,45,52 However, the high degree of heterogeneity (I2 = 84%) suggested a high variability among the studies depending on the hospital setting, type of surgery, and patients. Concerning morbidity, NMA provided some interesting information. First, AE and AE + IMT arms reduced the overall morbidity rate compared with NST. However, this effect remains uncertain due to imprecision, because the confidence interval crosses the minimal important difference. Moreover, the impact could be highly variable for the AE arm due to indirectness. Indeed, three studies32,39,47 enrolled only frailty subjects such as cirrhotic or high-risk patients. The estimated effect suggested a slight advantage for AE alone, even if none of the components or combinations has a statistically significant incremental effect. In other words, the overall complications represent a composite endpoint in which many complications are included, but only a few could be influenced by preoperative exercise. Analysis of major morbidity and mortality confirms this hypothesis. Complications requiring surgical, endoscopic, or radiological reintervention depended on surgical-related factors, such as anastomotic leak, septic events, or hemorrhage. All of these complications cannot be avoided by physical prehabilitation. Physical preoperative exercise could make patients more resistant to the potentially negative consequences of a complicated postoperative course. Still, this effect is challenging to capture by measuring the severity of complications or crude mortality rate. It could be recognized by estimating the failure to rescue rate (FTR), which represents the mortality rate calculated only among patients who experience major complications and depends on several factors, including the preexistent conditions of the patients.54 Unfortunately, none of the included studies reported FTR. Considering the pneumonia rate, AE + IMT and AE was the best approach with a clinically relevant effect. However, the imprecision, incoherence not evaluable, and indirectness (only for the AE arm) reduced the certainty of the results. Interestingly, the component analysis confirmed that the best plausible and statistically significant effect could be obtained by combining AE with IMT. These results did not surprise us: it is physiologically reasonable that increasing VO2 max and ventilatory capacity could avoid infectious respiratory complications after major abdominal surgery. A recent systematic review indicates that a significant percentage, up to 28%, of major abdominal surgical procedures could be complicated by pneumonia.55 The risk increases two or three times when poor lung function is preoperative present. Concerning LOS, the only approach among the best is the AE alone, whereas IMT alone and IMT + AE maintained a marginal effect, resulting in better than worst. These data are uncertain due to a miscellanea of bias, such as imprecision, incoherence, within-study bias, and heterogeneity. The component analysis suggested that the best combination could be AE + IMT with a potentially significant reduction of LOS up to 2 days. These results are credible from a physiopathological point of view, but they should be interpreted with prudence, because the LOS is a weak measure of efficacy. Several confounding factors, such as the age of patients, the type of healthcare system, the type of surgery, and other social factors, easily influence hospitalization. Other objective measures could be used to capture the effect of prehabilitation on the LOS, such as functional recovery, type of discharge (e.g., at home or with the need for further rehabilitation), or quality-of-life questionnaire. Unfortunately, these data are little or nothing available within the included studies.
The present study has some limitations. First, the included studies cover a relatively long period. Another limitation is the lack of a standardized definition of the outcomes not corrigible with rigorous data extraction (within-study bias). These biases can be limited by using all statistical instruments to capture the heterogeneity, inconsistency, and publication bias. The CINeMA approach measured the weight of bias, but they did not erase it. Finally, the local incoherence cannot be evaluated due to the absence of closed loops. Therefore, all studies were considered by default at risk using CINeMA and GRADE methodology.
Conclusions
The present study suggests that physical prehabilitation could play a role in patients who underwent major abdominal surgery, reducing minor or respiratory complications. This hypothesis seems to be coherent with the observed reduction of LOS, quantifiable in less than 2 days. Even if the ideal approach does not exist, CNMA indicates that the simultaneous use of AE and IMT could be more effective than AE or IMT alone in obtaining clinically relevant results. Further high-quality, randomized studies are needed to validate the routine use of physical prehabilitation. Indeed, physical prehabilitation could reduce the FTR, making the patients more resistant to the negative effects of major complications. Unfortunately, the FTR was never reported, and this remained only a speculative hypothesis. Nonetheless, our results could help design new trials, indicating the simultaneous use of AE + IMT in the intervention arm and the use of new outcomes, such as failure to rescue or functional recovery.
References
Kehlet H. Fast-track surgery: an update on physiological care principles to enhance recovery. Langenbecks Arch Surg. 2011;358:585–90.
McCulloch P, Ward J, Tekkis pp. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192–7.
Scott MJ, Baldini G, Fearon KC, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand. 2015;59:1212–31.
Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: enhanced recovery after surgery (ERAS®) Society recommendations. Br J Surg. 2014;101:1209–29.
Carli F, Gillis C, Scheede-Bergdahl C. Promoting a culture of prehabilitation for the surgical cancer patient. Acta Oncol. 2017;56:128–33.
Pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) recommendations 2019. World J Surg. 2020;44:2056–84.
Melloul E, Lassen K, Roulin D, et al. Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World J Surg. 2020;44:2056–84.
Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJE. Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg. 2019;43:1661–8.
Tsokani S, Seitidis G, Mavridis D. Component network meta-analysis in a nutshell. BMJ Evid Based Med. 2022 Jul 27:bmjebm-2021-111906. Epub head of print
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;3:e1003082.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE working group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;11:777–84.
Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106:420–1.
Clark J, Glasziou P, Del Mar C, et al. A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol. 2020;121:81–90.
Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-7.
Hultcrantz M, Rind D, Akl EA, et al. The grade working group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13.
Seitidis G, Tsokani S, Christogiannis C, Kontouli KM, Fyraridis A, Nikolakopoulos S, Veroniki AA, Mavridis D. Graphical tools for visualizing the results of network meta-analysis of multicomponent interventions. Res Synth Methods. 2022. https://doi.org/10.1002/jrsm.1617.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Method. 2015;15:58.
Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900.
Yepes-Nuñez JJ, Li SA, Guyatt G, et al. Development of the summary of findings table for network meta-analysis. J Clin Epidemiol. 2019;115:1–13.
Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomised controlled trials. J Clin Epidemiol. 1997;50:683–91.
Shim S, Yoon BH, Shin IS, et al. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047.
Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Schünemann H, Brożek J, Guyatt G, et al. Quality of evidence. In: Schünemann H, Brożek J, Guyatt G, Oxman A (eds). GRADE handbook. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 12 Aug 2022.
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082.
Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ, Scott S, Mayo NE. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97:1187–97.
Dronkers JJ, Lamberts H, Reutelingsperger IM, Naber RH, Dronkers-Landman CM, Veldman A, van Meeteren NL. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin Rehabil. 2010;24:614–22.
Kulkarni SR, Fletcher E, McConnell AK, Poskitt KR, Whyman MR. Pre-operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery: a randomised pilot study. Ann R Coll Surg Engl. 2010;92:700–7.
Kaibori M, Ishizaki M, Matsui K, Nakatake R, Yoshiuchi S, Kimura Y, Kwon AH. Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg. 2013;206:202–9.
Soares SM, Nucci LB, da Silva MM, Campacci TC. Pulmonary function and physical performance outcomes with preoperative physical therapy in upper abdominal surgery: a randomized controlled trial. Clin Rehabil. 2013;27:616–27.
Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, Liberman AS, Stein B, Charlebois P, Feldman LS, Carli F. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–47.
Jensen BT, Petersen AK, Jensen JB, Laustsen S, Borre M. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: a prospective randomized controlled trial. Scand J Urol. 2015;49:133–41.
Dunne DF, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ, Poston GJ, Palmer DH, Fenwick SW. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016;103:504–12.
Boden I, Skinner EH, Browning L, Reeve J, Anderson L, Hill C, Robertson IK, Story D, Denehy L. Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: pragmatic, double blinded, multicentre randomised controlled trial. BMJ. 2018;24(360):j5916.
Banerjee S, Manley K, Shaw B, Lewis L, Cucato G, Mills R, Rochester M, Clark A, Saxton JM. Vigorous intensity aerobic interval exercise in bladder cancer patients prior to radical cystectomy: a feasibility randomised controlled trial. Support Care Cancer. 2018;26:1515–23.
Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R, Momblán D, Balust J, Blanco I, Martínez-Pallí G. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. 2018;267:50–6.
Bousquet-Dion G, Awasthi R, Loiselle SÈ, Minnella EM, Agnihotram RV, Bergdahl A, Carli F, Scheede-Bergdahl C. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol. 2018;57:849–59.
Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. 2018;153:1081–9.
Valkenet K, Trappenburg JCA, Ruurda JP, Guinan EM, Reynolds JV, Nafteux P, Fontaine M, Rodrigo HE, van der Peet DL, Hania SW, Sosef MN, Willms J, Rosman C, Pieters H, Scheepers JJG, Faber T, Kouwenhoven EA, Tinselboer M, Räsänen J, Ryynänen H, Gosselink R, van Hillegersberg R, Backx FJG. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br J Surg. 2018;105:502–11.
Ausania F, Senra P, Meléndez R, Caballeiro R, Ouviña R, Casal-Núñez E. Prehabilitation in patients undergoing pancreaticoduodenectomy: a randomized controlled trial. Rev Esp Enferm Dig. 2019;111:603–8.
Karlsson E, Farahnak P, Franzén E, Nygren-Bonnier M, Dronkers J, van Meeteren N, Rydwik E. Feasibility of preoperative supervised home-based exercise in older adults undergoing colorectal cancer surgery: a randomized controlled design. PLoS One. 2019;14:e0219158.
Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, Stein B, Charlebois P, Ghitulescu G, Morin N, Jagoe T, Scheede-Bergdahl C, Minnella EM, Fiore JF Jr. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155:233–42.
PREPARE-ABC Trial Collaborative. SupPoRtive exercise programmes for accelerating REcovery after major ABdominal cancer surgery trial (PREPARE-ABC): pilot phase of a multicentre randomised controlled trial. Colorectal Dis. 2021;23:3008–22.
Moug SJ, Mutrie N, Barry SJE, Mackay G, Steele RJC, Boachie C, Buchan C, Anderson AS. Prehabilitation is feasible in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy and may minimize physical deterioration: results from the REx trial. Colorectal Dis. 2019;21:548–62.
Waller E, Sutton P, Rahman S, Allen J, Saxton J, Aziz O. Prehabilitation with wearables versus standard of care before major abdominal cancer surgery: a randomised controlled pilot study (trial registration: NCT04047524). Surg Endosc. 2022;36:1008–17.
Allen SK, Brown V, White D, King D, Hunt J, Wainwright J, Emery A, Hodge E, Kehinde A, Prabhu P, Rockall TA, Preston SR, Sultan J. Multimodal prehabilitation during neoadjuvant therapy prior to esophagogastric cancer resection: effect on cardiopulmonary exercise test performance, muscle mass and quality of life-a pilot randomized clinical trial. Ann Surg Oncol. 2022;29:1839–50.
Berkel AEM, Bongers BC, Kotte H, Weltevreden P, de Jongh FHC, Eijsvogel MMM, Wymenga M, Bigirwamungu-Bargeman M, van der Palen J, van Det MJ, van Meeteren NLU, Klaase JM. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg. 2022;275:e299-306.
Gloor S, Misirlic M, Frei-Lanter C, Herzog P, Müller P, Schäfli-Thurnherr J, Lamdark T, Schregel D, Wyss R, Unger I, Gisi D, Greco N, Mungo G, Wirz M, Raptis DA, Tschuor C, Breitenstein S. Prehabilitation in patients undergoing colorectal surgery fails to confer reduction in overall morbidity: results of a single-center, blinded, randomized controlled trial. Langenbecks Arch Surg. 2022;407:897–907.
Onerup A, Andersson J, Angenete E, Bock D, Börjesson M, Ehrencrona C, Fagevik Olsén M, Larsson PA, de la Croix H, Wedin A, Haglind E. Effect of short-term homebased pre- and postoperative exercise on recovery after colorectal cancer surgery (PHYSSURG-C): a randomized clinical trial. Ann Surg. 2022;275:448–55.
Woodfield JC, Clifford K, Wilson GA, Munro F, Baldi JC. Short-term high-intensity interval training improves fitness before surgery: a randomized clinical trial. Scand J Med Sci Sports. 2022;32:856–65.
Rosero EB, Modrall JG, Joshi GP. Failure to rescue after major abdominal surgery: the role of hospital safety net burden. Am J Surg. 2020;220:1023–30.
Chughtai M, Gwam CU, Mohamed N, Khlopas A, Newman JM, Khan R, Nadhim A, Shaffiy S, Mont MA. The epidemiology and risk factors for postoperative pneumonia. J Clin Med Res. 2017;9:466–75.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. The authors did not receive funds or grants for the manuscript. The authors disclose any commercial interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
All authors declared that they did not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ricci, C., Alberici, L., Serbassi, F. et al. Physical Prehabilitation in Patients who Underwent Major Abdominal Surgery: A Comprehensive Systematic Review and Component Network Meta-Analysis Using GRADE and CINeMA Approach. Ann Surg Oncol 31, 1725–1738 (2024). https://doi.org/10.1245/s10434-023-14632-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14632-8