Abstract
Background
A post-hoc analysis of ABC trials included 34 patients with liver-confined unresectable intrahepatic cholangiocarcinoma (iCCA) who received systemic chemotherapy with gemcitabine and cisplatin (gem-cis). The median overall survival (OS) was 16.7 months and the 3-year OS was 2.8%. The aim of this study was to compare patients treated with systemic gem-cis versus hepatic arterial infusion pump (HAIP) chemotherapy for liver-confined unresectable iCCA.
Methods
We retrospectively collected consecutive patients with liver-confined unresectable iCCA who received gem-cis in two centers in the Netherlands to compare with consecutive patients who received HAIP chemotherapy with or without systemic chemotherapy in Memorial Sloan Kettering Cancer Center.
Results
In total, 268 patients with liver-confined unresectable iCCA were included; 76 received gem-cis and 192 received HAIP chemotherapy. In the gem-cis group 42 patients (55.3%) had multifocal disease compared with 141 patients (73.4%) in the HAIP group (p = 0.023). Median OS for gem-cis was 11.8 months versus 27.7 months for HAIP chemotherapy (p < 0.001). OS at 3 years was 3.5% (95% confidence interval [CI] 0.0–13.6%) in the gem-cis group versus 34.3% (95% CI 28.1–41.8%) in the HAIP chemotherapy group. After adjusting for male gender, performance status, baseline hepatobiliary disease, and multifocal disease, the hazard ratio (HR) for HAIP chemotherapy was 0.27 (95% CI 0.19–0.39).
Conclusions
This study confirmed the results from the ABC trials that survival beyond 3 years is rare for patients with liver-confined unresectable iCCA treated with palliative gem-cis alone. With HAIP chemotherapy, one in three patients was alive at 3 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Intrahepatic cholangiocarcinoma (iCCA) is the second most prevalent primary liver cancer after hepatocellular carcinoma. The incidence of iCCA in Western countries is approximately 1 per 100,000 and it is increasing rapidly.1,2,3 The majority of iCCAs present at a locally advanced, unresectable stage with limited treatment options due to its late manifestation.4,5 Therefore, most patients are only eligible for palliative systemic treatment.6 The median overall survival (OS) of patients with unresectable iCCA is about 5 months when no systemic treatment is administered.7,8
Palliative systemic treatment for iCCA is typically investigated in studies including all patients with biliary cancers: cholangiocarcinoma (intrahepatic, perihilar, and distal) and gallbladder cancer. The most common regimen for advanced biliary cancers is the combination of gemcitabine and cisplatin (gem-cis), which offers a small OS benefit over gemcitabine monotherapy (11.7 versus 8.1 months, respectively, HR 0.64, p < 0.001).2 Patients with advanced iCCA mostly have locally advanced rather than distant metastatic disease.5,9 A post-hoc analysis of 34 patients with liver-confined unresectable iCCA, treated with gem-cis in the ABC trials, found a median OS of 16.7 months (95% CI 8.2–20.0) and 3-year OS of 2.8%.10 These results are a benchmark for any additional locoregional treatment.
Regional treatments are increasingly used to improve OS in liver-confined unresectable iCCA. The main rationale for locoregional treatment of locally advanced iCCA is that most patients die from progressive disease in the liver with biliary obstruction and liver failure.11 Liver-directed therapy via a hepatic arterial infusion pump (HAIP) enables the delivery of high-dose chemotherapy (floxuridine) directly into the liver. A continuous flow of intra-arterial chemotherapy is delivered in the hepatic artery via a surgically implantable subcutaneous pump with a catheter in the gastroduodenal artery (GDA), which is a side branch of the hepatic artery. Floxuridine has a 95% first-pass effect, which allows for a 400-fold intra-tumoral concentration compared with systemic administration, without systemic side effects. Three phase II trials from Memorial Sloan Kettering Cancer Center (MSKCC) showed promising results with response rates over 50% and median OS ranging from 25.0 to 30.8 months.12,13,14
The aim of this study was to compare OS after gem-cis versus hepatic arterial infusion pump (HAIP) chemotherapy with or without systemic chemotherapy in patients with liver-confined unresectable iCCA.
Methods
Cohort Selection
The study protocol of this multicenter retrospective study was approved by the Institutional Review Board (IRB) of Erasmus MC Cancer Institute Rotterdam (MEC-2021-0501) prior to data collection and processing. Informed consent was waived by the IRB. Data were retrieved from existing medical records.
All consecutive patients diagnosed with liver-confined unresectable iCCA, confirmed by a biopsy, or determined at a multidisciplinary team meeting, were identified. The patients that were treated with systemic gem-cis in Erasmus MC Cancer Institute (Rotterdam, the Netherlands) and Amsterdam UMC (Amsterdam, the Netherlands), were identified between January 2014 and December 2019. The patients that were treated with HAIP floxuridine in Memorial Sloan Kettering Cancer Center (New York, United States), were identified between January 2000 and December 2019. Patients in both groups were excluded if they had undergone prior liver surgery or had distant metastases at time of diagnosis. Patients with locoregional lymph node metastasis (pathologically proven or based on imaging) were not excluded. In the gem-cis group, patients were excluded if they had received first-line chemotherapeutic regimens other than gem-cis. Patients were followed until death or the date they were lost to follow-up.
The following data were extracted for each patient: demographics, baseline hepatobiliary disease, Eastern Cooperative Oncology Group (ECOG) performance status, prior treatment, drainage, date of diagnosis, tumor distribution, cancer antigen (CA) 19-9 serum level at start of treatment, concurrent systemic treatment administration, and date of death or last follow-up. Second-line systemic treatment data were not collected. Tumor distribution included the presence of locoregional lymph node metastasis (pathologically proven or based on imaging), diameter of the largest tumor, and number of tumors. Subsequent locoregional treatments after gem-cis administration or HAIP chemotherapy were also collected.
Variable Definitions
Liver-confined unresectable iCCA was defined as disease confined to the liver that is unresectable owing to tumor location and/or multifocal involvement and/or locoregional lymph node metastasis. The primary endpoint OS was defined as the time between date of diagnosis and date of death or last follow-up. Resection rate was defined as the percentage of patients who underwent surgery after initial treatment.
Patients’ performance status and CA 19-9 serum level were those measured at the closest time before the start of initial treatment. Multifocal disease was defined as more than one lesion in the liver on imaging, whether it concerned intrahepatic metastases or satellites surrounding the largest lesion. Locoregional lymph nodes were positive or negative for cancer based on pathology results of excisions and biopsies. Locoregional lymph nodes were considered suspicious for cancer on imaging, as defined by a short axis larger than 10 mm and/or a necrotic center of the lymph node assessed by an expert radiologist.
Statistical Analysis
Continuous variables are presented as mean with standard deviation (SD) or medians with interquartile range (IQR) or range. Differences were tested with the help of Student’s t-test or the Mann-Whitney test, depending on the variable’s distribution. Categorical variables are presented as proportions with corresponding frequencies, and differences were tested with the chi-square (χ2) or Fisher exact test, whichever was appropriate. Missing values were excluded from analysis.
OS was estimated using the Kaplan–Meier method. Patients lost to follow-up were censored. Cox proportional-hazards (CPH) models were used to assess associations between the primary endpoint OS and several variables, including patient demographics and tumor characteristics. The multivariate analysis used the backward selection regression method. Variables that were statistically significant on univariate analysis (p < 0.20) were included in the multivariate model along with known relevant variables. Outcomes are presented as hazard ratio (HR) with 95% confidence intervals (CI). Pre-specified sensitivity analyses were performed for first-line (i.e., without previous systemic chemotherapy) and second-line (i.e., with previous first-line systemic chemotherapy) HAIP chemotherapy. Within the subgroup of patients receiving first-line HAIP chemotherapy, patients with and without concurrent systemic chemotherapy were compared. A sensitivity analysis was also performed by adding prognostic factors from the literature (i.e., tumor diameter, serum level CA 19-9) to the multivariate model.
Analyses were performed with the statistical software program R (R Core team, 2021: version 4.1.0, Vienna, Austria) using the package ‘survival’ and the statistical software program IBM SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). All tests were two-tailed and statistical significance was defined as p < 0.05.
Results
In total, 268 patients with liver-confined unresectable iCCA were included: 76 in the gem-cis group and 192 in the HAIP chemotherapy group. Of the 76 patients who received gem-cis, 50 were treated in Erasmus MC Cancer Institute and 26 in Amsterdam UMC (Fig. 1). Baseline characteristics are presented in Table 1. In the gem-cis group, 42 patients (55.3%) had multifocal disease compared with 141 patients (73.4%) in the HAIP group (p = 0.023). The diameter of the largest tumor was similar in both groups (8.5 vs 8.4 cm, p = 0.833). ECOG performance status did not differ between the groups (ECOG 0 or 1, 95.6% vs 97.1%, p = 0.214). Baseline hepatobiliary disease was more common in the gem-cis group (18.4% vs 5.2%, p = 0.001). Most patients (n = 134, 69.8%) received HAIP chemotherapy as first-line treatment; 58 patients (30.2%) in the HAIP group received prior first-line systemic chemotherapy. HAIP chemotherapy was combined with systemic chemotherapy in 138 patients (71.9%), with regimes including gemcitabine with cisplatin or oxaliplatin, gemcitabine monotherapy, and irinotecan (Table 2). Forty-two patients (21.9%) received HAIP as first-line treatment without concurrent systemic treatment.
Survival
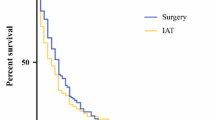
Median OS for gem-cis was 11.8 months (95% CI 10.3–13.9 months) versus 27.7 months (95% CI 23.7–30.6 months) for HAIP chemotherapy (p < 0.001). Three-year OS was 3.5% (95% CI 0.0–13.6%) in the gem-cis group and 34.3% (95% CI 28.1–41.8%) in the HAIP chemotherapy group. Five-year OS was 0% in the gem-cis group and 15.1% (95% CI 10.5–21.8%) in the HAIP chemotherapy group (Fig. 2).
The subgroup of patients with first-line HAIP chemotherapy had a median OS of 27.2 months (95% CI 23.1–30.3 months) versus 30.0 months (95% CI 23.7–39.8 months) for second-line HAIP chemotherapy. Three-year OS was 32.2% (95% CI 25.1–41.3%) for patients with first-line HAIP chemotherapy versus 39.3% (95% CI 28.4–54.6%) for second-line HAIP chemotherapy (Fig. 3). In the subgroup of patients who received first-line HAIP with concurrent systemic treatment, the median OS was 26.4 months versus 29.4 months for patients without concurrent systemic treatment (p = 0.7) (Fig. 4). Three-year OS was 34% (95% CI 25.4–45.3%) for patients who received concurrent systemic treatment and 30% (95% CI 18.7–48.2%) for patients without concurrent systemic treatment.
Independent Poor Prognostic Factors
Table 3 shows the independent poor prognostic factors including male gender (HR 1.45; 95% CI 1.09–1.94; p = 0.01), poor ECOG performance status (2 vs 0, HR 4.22; 95% CI 1.91–9.32; p < 0.001), baseline hepatobiliary disease (HR 2.26; 95% CI 1.33–3.84; p = 0.003), and multifocal disease (≥ 4 vs 1 lesion, HR 1.51; 95% CI 1.08–2.12; p = 0.02). After adjusting for these four poor prognostic factors, HAIP chemotherapy was associated with superior OS compared with systemic chemotherapy alone (HR 0.27; 95% CI 0.19–0.39; p < 0.001). After adjusting for additional known poor prognostic factors based on the literature (i.e., tumor diameter ≥ 5 cm, serum level CA 19-9 ≥ 500 kU/l) the adjusted HR for HAIP chemotherapy compared with gem-cis was 0.33 (95% CI 0.22–0.50; p < 0.001).
Additional Locoregional Treatments
Subsequent locoregional treatment was performed in 3 patients (3.9%) after gem-cis alone compared with 18 patients (9.4%) after HAIP chemotherapy (p = 0.14; Table 2). Locoregional treatment mostly involved surgical resection and was performed in 14 patients (5.2%); one patient (1.3%) receiving gem-cis alone and 13 patients (6.8%) receiving HAIP chemotherapy. OS after resection was 61.5% at 3 years and 44.9% at 5 years. Ablation (i.e., radiofrequency ablation, microwave ablation, or irreversible electroporation) was performed in three patients; one patient (1.3%) after gem-cis and two patients (1.0%) after HAIP chemotherapy. Transarterial chemoembolization (TACE) was performed in two patients (2.6%) after gem-cis and 3 patients (1.6%) after HAIP chemotherapy. External radiotherapy was only performed in one patient (0.5%) after HAIP chemotherapy. Selective internal radiation therapy (SIRT) was not performed.
Discussion
In this study of patients with liver-confined unresectable iCCA, we found that patients had a median OS of 11.8 months when treated with gem-cis alone versus 27.7 months when treated with HAIP chemotherapy with or without concurrent systemic chemotherapy (p < 0.001). Three-year OS was 3.5% with gem-cis alone versus 34.3% with HAIP chemotherapy. Within the HAIP group, OS was similar after first-line and second-line HAIP chemotherapy. Moreover, no difference in OS could be demonstrated between patients receiving first-line HAIP chemotherapy with or without concurrent systemic chemotherapy. Independent poor prognostic factors for patients with liver-confined unresectable iCCA were male gender, poor performance status (ECOG 2), baseline hepatobiliary disease, and multifocal disease. HAIP chemotherapy remained an independent favorable prognostic factor (HR 0.33; 95% CI 0.22–0.50; p < 0.001) after adjusting for all known confounders.
A SEER population-based cohort study of 5616 patients with advanced intrahepatic cholangiocarcinoma reported a median OS of about 5 months and a 3-year OS of about 3%.8 Patients who received gem-cis for liver-confined unresectable iCCA in the ABC trials had a median OS of 16.7 months, with no survivors beyond 3 years.2 The median OS after gem-cis in the present study was 11.8 months with a 3-year OS of 3.5%. This median OS was lower compared with the ABC trials, a difference that likely reflects the strict inclusion criteria. In a previous study, we found that about 40% of patients who received gem-cis for advanced iCCA in the “real world” did not fulfill inclusion criteria of the ABC trials.15 Regardless of baseline patient characteristics, however, the 3-year OS in any advanced iCCA cohort receiving palliative systemic chemotherapy has been close to zero.
The main rationale for locoregional treatment for advanced iCCA is that most patients die from progressive disease in the liver with biliary obstruction and liver failure. In a large cohort study of 362 patients with iCCA at MD Anderson, the authors found that in patients who were treated with palliative systemic chemotherapy (n = 99), 71 (72%) died from liver failure secondary to local tumor progression.11 The impact of local (rather than distant) tumor progression may also explain why the survival curves of patients with liver-confined unresectable iCCA and patients with extrahepatic metastases were overlapping in the subgroup of patients with iCCA who received gem-cis in the ABC trials.10
Three phase II trials investigated the combination of systemic and HAIP chemotherapy for locally advanced iCCA.12,13,14 These trials consistently reported a partial response rate of about 50%, a median OS of about 25 months, and a 3-year OS rate of about 35%. A recent systematic review and meta-analysis of HAIP chemotherapy with floxuridine for unresectable iCCA included 154 patients from four studies and found a median OS of 29.0 months (range 25.0–39), a 3-year OS of 39.5% (95% CI 31.5–47.4), and a 5-year OS of 9.7% (95% CI 0.0–23.4).16 In particular, the 3-year OS of 35% is an enormous improvement compared with negligible 3-year OS with systemic chemotherapy alone. In our study, most patients (152/192, 79%) who received HAIP chemotherapy also received systemic chemotherapy before or during HAIP chemotherapy. It is unlikely that systemic chemotherapy alone was responsible for the favorable survival outcomes in the HAIP group, considering that 3-year OS was not observed with systemic chemotherapy alone in the ABC trials.10 We could not demonstrate a difference in OS between patients who received first-line HAIP with or without systemic treatment. The sample size, however, was low and we believe that HAIP chemotherapy should be given in addition to systemic treatment. HAIP chemotherapy has no systemic toxicity and the complication rates (e.g., biliary sclerosis and pump pocket infection) are low in experienced hands.17
Liver-directed therapies are recommended by international guidelines for multifocal or locally advanced (i.e., unresectable) iCCA.18,19,20,21 iCCA lesions are often too large for percutaneous ablation. Several percutaneous intra-arterial approaches have been investigated, including transarterial chemoembolization and selective internal radiotherapy (SIRT). A phase II trial investigated a combination of first-line SIRT and systemic gem-cis in locally advanced iCCA patients (n = 41) with a median OS of 22 months (95% CI 14–52 months).22 These results are promising, but appear to be inferior to HAIP chemotherapy. A phase III trial investigating first-line SIRT in patients with liver-confined iCCA was prematurely closed due to lack of accrual (NCT02807181). The main advantage of HAIP chemotherapy compared with SIRT is that lesions are treated across the entire liver, regardless of the number of lesions and whether they are visible on imaging.
This study has several limitations. First, the comparison between the two treatment groups was not randomized and patients were included over a long period of time. We adjusted for known poor prognostic factors, but unknown confounders may have biased the results. For example, about 5% of patients with liver-confined iCCA on imaging have occult peritoneal metastases. These patients would not be eligible for HAIP chemotherapy when metastatic disease is detected at surgical exploration for pump implantation. In the gem-cis cohort these metastases would remain undetected. However, any residual selection bias cannot explain a 3-year OS after HAIP chemotherapy of 1 in 3 patients versus no 3-year OS in the gem-cis group of the ABC trials. Second, data on genomic alterations were missing for most patients, while these alterations are of increasing importance for targeted therapy (i.e., isocitrate dehydrogenase and fibroblast growth factor receptor alterations) and prognosis.23,24,25,26,27,28,29 In a phase II study, 108 patients with previously treated advanced CCA with FGFR2 fusions or rearrangements received infigratinib. The median OS was only 12 months, but the 3-year OS was about 25%.25 In the FOENIX-CCA2 study, 103 patients with iCCA harboring FGFR2 fusion or rearrangements received futibatinib. The median OS was 20.0 months.28 In the FIGHT-202 trial, 107 patients with previously treated advanced CCA with FGFR2 fusions or rearrangements received pemigatinib. The median OS was 21.1 months.29 As a result of these promising outcomes, three randomized studies in the first-line setting are ongoing (NCT03773302, NCT04093362, NCT03656536). Finally, HAIP chemotherapy is currently offered in only about 60 centers worldwide. It is a complex treatment requiring close collaboration of a multidisciplinary team.
In conclusion, patients with liver-confined unresectable iCCA had a 3-year OS of 34.3% after HAIP chemotherapy compared with 3.5% when treated with systemic chemotherapy alone.
References
Park J, Kim MH, Kim KP, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver. 2009;3(4):298–305.
Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. New Engl J Med. 2010;362(14):1273–81.
https://iknl.nl/nkr-cijfers.https://nkr-cijfers.iknl.nl/viewer/incidentie-per-jaar?language=nl&viewerId=495e4f2b-5f7b-456c-a0ac-b8d495301edf.
de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–5.
Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96.
Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008;10(2):77–82.
Massani M, Nistri C, Ruffolo C, et al. Intrahepatic chemotherapy for unresectable cholangiocarcinoma: review of literature and personal experience. Updates Surg. 2015;67(4):389–400.
Mody K, Antwi SO, Hodge DO, Ailawadhi S, Roberts L, Bekaii-Saab T. A SEER-based multi-ethnic picture of advanced intrahepatic cholangiocarcinoma in the United States pre- and post-the advent of gemcitabine/cisplatin. J Gastrointest Oncol. 2018;9(6):1063–73.
Hahn F, Müller L, Mähringer-Kunz A, et al. Distant metastases in patients with intrahepatic cholangiocarcinoma: does location matter? A retrospective analysis of 370 patients. J Oncol. 2020;2020:7195373.
Lamarca A, Ross P, Wasan HS, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020;112(2):200–10.
Yamashita S, Koay EJ, Passot G, et al. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: a comprehensive analysis of 362 consecutive patients. Cancer. 2017;123(8):1354–62.
Cercek A, Boerner T, Tan BR, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2019;6(1):60–7.
Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20(9):1589–95.
Kemeny NE, Jarnagin WR, Capanu M, et al. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol. 2011;29(7):884–9.
Dierks J, Gaspersz MP, Belkouz A, et al. Translating the ABC-02 trial into daily practice: outcome of palliative treatment in patients with unresectable biliary tract cancer treated with gemcitabine and cisplatin. Acta Oncol. 2018;57(6):807–12.
Holster JJ, El Hassnaoui M, Franssen S, et al. Hepatic arterial infusion pump chemotherapy for unresectable intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2022. https://doi.org/10.1245/s10434-022-11439-x.
Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201(1):57–65.
Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122(5):758–65.
Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: Cancer J Clin. 2017;67(2):93–9.
Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–89.
Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):669–80.
Edeline J, Touchefeu Y, Guiu B, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6(1):51–9.
Boerner T, Drill E, Pak LM, et al. Genetic determinants of outcome in intrahepatic cholangiocarcinoma. Hepatology. 2021;74(3):1429–44.
Abou-Alfa GK, Macarulla Mercade T, Javle M, et al. LBA10_PR: ClarIDHy—a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Ann Oncol. 2019;30:v872–3.
Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):276–82.
Vogel A, Sahai V, Hollebecque A, et al. LBA40 - FIGHT-202: a phase II study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA). Ann Oncol. 2019;30:v876.
Marin JJG, Prete MG, Lamarca A, et al. Current and novel therapeutic opportunities for systemic therapy in biliary cancer. Br J Cancer. 2020;123(7):1047–59.
Lipika G, Funda M-B, Antoine H, et al. Updated results of the FOENIX-CCA2 trial: efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/rearrangements. J Clin Oncol. 2022;40((16_suppl)):4009.
Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84.
Acknowledgements
Stijn Franssen received support from the Dutch Cancer Society (KWF) on project number 12077. Joshua Jolissaint received support from the Weill Cornell Medical College (WCMC). Clinical and Translational Science Center (CTSC) funded by NIH/NCATS UL1TR002384.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. James Harding. Research Support to Institution: Bristol Myers Squibb, Boehringer Ingelheim, CytomX, Debiopharm, Eli Lilly, Genoscience, Incyte, Loxo @ Lilly, Novartis, Polaris, Pfizer, Zymeworks, and Yiviva. Consulting/Advisory: Adaptimmune, Astrazenica, Bristol Myers Squibb, Exelexis, Elevar, Eisai, Genoscience (uncompensated), Hepion, Imvax, Merck (DSMB) Medivir, QED, Tyra, and Zymeworks (uncompensated).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franssen, S., Holster, J.J., Jolissaint, J.S. et al. Gemcitabine with Cisplatin Versus Hepatic Arterial Infusion Pump Chemotherapy for Liver-Confined Unresectable Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 31, 115–124 (2024). https://doi.org/10.1245/s10434-023-14409-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14409-z