Abstract
Introduction
There are no approved locoregional therapies for peritoneal carcinomatosis from gastric adenocarcinoma (GA). Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS–HIPEC) represents a potential treatment for advanced GA with isolated peritoneal metastasis.
Patients and Methods
Two separate single-institution phase II, single-arm studies evaluating CRS–HIPEC using cisplatin with mitomycin C (NIH: NCT03092518, MDACC: NCT02891447) in patients with GA and confirmed peritoneal metastasis were analyzed. The primary endpoint of each trial was overall survival (OS). Clinical, pathologic, and treatment variables were analyzed for association with outcomes.
Results
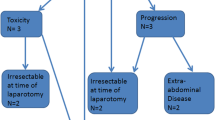
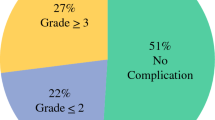
Over 4 years, 41 patients with peritoneal carcinomatosis from GA underwent CRS–HIPEC. All patients had synchronous peritoneal metastasis and received systemic chemotherapy as front-line therapy. A total of 23 patients also received laparoscopic HIPEC prior to open CRS–HIPEC. The majority (63%, n = 26) were male, and median PCI score at CRS–HIPEC was 2. Median OS was 24.9 months from diagnosis and 14.4 months from CRS–HIPEC. Three-year OS was 25% from diagnosis and 22% from CRS–HIPEC. Median RFS was 7.4 months. The rate of 30-day Clavien–Dindo grade ≥ 3 complications was 32%; specifically, the rate of anastomotic leak was 22%. Multivariable analysis identified the number of pathologically positive lymph nodes as an independent predictor of postoperative OS.
Conclusions
In patients with gastric adenocarcinoma and isolated peritoneal metastasis treated with CRS–HIPEC, 3-year OS was 22% from CRS–HIPEC, and complications were common. The number of pathologic lymph node metastases was inversely correlated with overall survival. Further investigation of CRS–HIPEC for GA should include patient selection based on response to systemic chemotherapy or incorporate novel intraperitoneal treatment strategies.


Similar content being viewed by others
References
Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38.
Ajani JA, et al. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036.
Ikoma N, et al. Patterns of initial recurrence in gastric adenocarcinoma in the era of preoperative therapy. Ann Surg Oncol. 2017;24(9):2679–87.
Meneses Medina MI, et al. Morbidity associated to advanced gastric cancer and its impact on overall survival. J Clin Oncol. 2020;38(4_suppl):327–327.
Alexander HR Jr, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery. 2013;153(6):779–86.
Armstrong DK, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43.
Chua TC, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–56.
Badgwell B, et al. A phase II trial of cytoreduction, gastrectomy, and hyperthermic intraperitoneal perfusion with chemotherapy for patients with gastric cancer and carcinomatosis or positive cytology. Ann Surg Oncol. 2021;28(1):258–64.
Rudloff U, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110(3):275–84.
Marano L, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer with synchronous peritoneal metastases: multicenter Study of ‘Italian peritoneal surface malignancies oncoteam-S.I.C.O.’ Ann Surg Oncol. 2021;28(13):9060–70.
Bonnot PE, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol. 2019;37(23):2028–40.
Rau B, et al. Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2020;23(1):11–22.
Brown ZJ, et al. Heated intraperitoneal chemotherapy and gastrectomy for gastric cancer in the U.S.: the time is now. J Gastrointest Oncol. 2017;8(6):1109–13.
Badgwell B, et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol. 2017;24(11):3338–44.
Blumenthaler AN, et al. Laparoscopic HIPEC for low-volume peritoneal metastasis in gastric and gastroesophageal adenocarcinoma. Ann Surg Oncol. 2020;27(13):5047–56.
Badgwell B, et al. Lessons learned from a phase II clinical trial of laparoscopic HIPEC for gastric cancer. Surg Endosc. 2018;32(1):512.
Newhook TE, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy is safe for patients with peritoneal metastases from gastric cancer and may lead to gastrectomy. Ann Surg Oncol. 2019;26(5):1394–400.
Manzanedo I, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer with peritoneal carcinomatosis: multicenter study of Spanish group of peritoneal oncologic surgery (GECOP). Ann Surg Oncol. 2019;26(8):2615–21.
Rau B, et al. 1376O The effect of hyperthermic intraperitoneal chemotherapy (HIPEC) upon cytoreductive surgery (CRS) in gastric cancer (GC) with synchronous peritoneal metastasis (PM): a randomized multicentre phase III trial (GASTRIPEC-I-trial). Ann Oncol. 2021;32:S1040.
Mezhir JJ, et al. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17(12):3173–80.
Jamel S, et al. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. Gastric Cancer. 2018;21(1):10–8.
Al-Batran S-E, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57.
Tidadini F, et al. Effect of pressurized intraperitoneal aerosol chemotherapy on the survival rate of patients with peritoneal carcinomatosis of gastric origin. J Gastrointest Cancer. 2022. https://doi.org/10.1007/s12029-021-00698-8.
Ishigami H, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018;36(19):1922–9.
Ikoma N, et al. Nodal downstaging in gastric cancer patients: promising survival if ypN0 is achieved. Ann Surg Oncol. 2018;25(7):2012–7.
Rihuete Caro C, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol. 2018;44(11):1805–10.
Wang R, et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat Med. 2021;27(1):141–51.
Pectasides E, et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(1):37–48.
Gotze TO, et al. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma: the phase III “PREVENT”- (FLOT9) trial of the AIO /CAOGI /ACO. BMC Cancer. 2021;21(1):1158.
Glehen O, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183.
Acknowledgement
We thank Dr. Martha Quezado and Dr. Markku Miettinen of the NIH Department of Pathology. We thank the NIH Molecular Pathology department for performing NGS. We thank Paul Juneau from the NIH Division of Library Services for statistical support.
Funding
This research was supported by the Intramural Research Program, National Institutes of Health, National Cancer Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
Dr. Alisa Blumenthaler participated in a T32 research fellowship funded by the NIH from July 2019–June 2021, including work on this manuscript. The funder did not have any influence over the work included in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Green, B.L., Blumenthaler, A.N., Gamble, L.A. et al. Cytoreduction and HIPEC for Gastric Carcinomatosis: Multi-institutional Analysis of Two Phase II Clinical Trials. Ann Surg Oncol 30, 1852–1860 (2023). https://doi.org/10.1245/s10434-022-12761-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12761-0