Abstract
Background
Lymph node status is vital for prognosis and treatment decisions for esophageal squamous cell carcinoma (ESCC). This study aimed to construct and evaluate an optimal radiomics-based method for a more accurate evaluation of individual regional lymph node status in ESCC and to compare it with traditional size-based measurements.
Methods
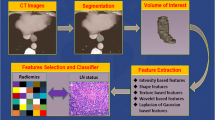
The study consecutively collected 3225 regional lymph nodes from 530 ESCC patients receiving upfront surgery from January 2011 to October 2015. Computed tomography (CT) scans for individual lymph nodes were analyzed. The study evaluated the predictive performance of machine-learning models trained on features extracted from two-dimensional (2D) and three-dimensional (3D) radiomics by different contouring methods. Robust and important radiomics features were selected, and classification models were further established and validated.
Results
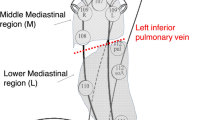
The lymph node metastasis rate was 13.2% (427/3225). The average short-axis diameter was 6.4 mm for benign lymph nodes and 7.9 mm for metastatic lymph nodes. The division of lymph node stations into five regions according to anatomic lymph node drainage (cervical, upper mediastinal, middle mediastinal, lower mediastinal, and abdominal regions) improved the predictive performance. The 2D radiomics method showed optimal diagnostic results, with more efficient segmentation of nodal lesions. In the test set, this optimal model achieved an area under the receiver operating characteristic curve of 0.841–0.891, an accuracy of 84.2–94.7%, a sensitivity of 65.7–83.3%, and a specificity of 84.4–96.7%.
Conclusions
The 2D radiomics-based models noninvasively predicted the metastatic status of an individual lymph node in ESCC and outperformed the conventional size-based measurement. The 2D radiomics-based model could be incorporated into the current clinical workflow to enable better decision-making for treatment strategies.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman DJ. Global cancer statistics. CA A Cancer J Clin. 2011;61:69–90.
Sugawara K, Yamashita H, Uemura Y, et al. Numeric pathologic lymph node classification shows prognostic superiority to topographic pN classification in esophageal squamous cell carcinoma. Surgery. 2017;162:846–56.
Gabriel E, Attwood K, Du W, et al. Association between clinically staged node-negative esophageal adenocarcinoma and overall survival benefit from neoadjuvant chemoradiation. JAMA Surg. 2016;151:234–45.
Rice TW, Ishwaran H, Hofstetter WL, et al. Esophageal cancer: associations with (pN+) lymph node metastases. Ann Surg. 2017;265:122–9.
Kutup A, Nentwich MF, Bollschweiler E, Bogoevski D, Izbicki JR, Hölscher AH. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg. 2014;260:1016–22.
Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46–50.
D’Journo XB. Clinical implication of the innovations of the 8(th) edition of the TNM classification for esophageal and esophago-gastric cancer. J Thorac Dis. 2018;10(Suppl 22):S2671–81.
Choi J, Kim SG, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc. 2010;24:1380–6.
Kato H, Kuwano H, Nakajima M, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer. 2002;94:921–8.
Li J, Chen S, Zhu G. Comparative study of computed tomography (CT) and pathological diagnosis toward mediastinal lymph node metastasis in esophageal carcinoma. Rev Assoc Med Bras. 2018;64:170–4.
Takemura M, Osugi H, Takada N, Kinoshita H, Higashino M. Prognostic factors in patients with squamous oesophageal cancer associated with solitary lymph node metastasis after oesophagectomy and extended lymphadenectomy. Oncol Rep. 2003;10:75–80.
Cao Q, Li Y, Li Z, An D, Li B, Lin Q. Development and validation of a radiomics signature on differentially expressed features of (18)F-FDG PET to predict treatment response of concurrent chemoradiotherapy in thoracic esophagus squamous cell carcinoma. Radiother Oncol. 2020;146:9–15.
Hu Y, Xie C, Yang H, et al. Assessment of intratumoral and peritumoral computed tomography radiomics for predicting pathological complete response to neoadjuvant chemoradiation in patients with esophageal squamous cell carcinoma. JAMA Netw Open. 2020;3:e2015927.
Yang Z, He B, Zhuang X, et al. CT-based radiomic signatures for prediction of pathologic complete response in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. J Radiat Res. 2019;60:538–45.
Beukinga RJ, Hulshoff JB, Mul VEM, et al. Prediction of response to neoadjuvant chemotherapy and radiation therapy with baseline and restaging (18)F-FDG PET imaging biomarkers in patients with esophageal cancer. Radiology. 2018;287:983–92.
Hou Z, Ren W, Li S, et al. Radiomic analysis in contrast-enhanced CT: predict treatment response to chemoradiotherapy in esophageal carcinoma. Oncotarget. 2017;8:104444–54.
Beukinga RJ, Hulshoff JB, van Dijk LV, et al. Predicting response to neoadjuvant chemoradiotherapy in esophageal cancer with textural features derived from pretreatment (18)F-FDG PET/CT imaging. J Nuclear Med. 2017;58:723–9.
Qiu Q, Duan J, Deng H, et al. Development and validation of a radiomics nomogram model for predicting postoperative recurrence in patients with esophageal squamous cell cancer who achieved pCR after neoadjuvant chemoradiotherapy followed by surgery. Front Oncol. 2020;10:1398.
Chen YH, Lue KH, Chu SC, et al. Combining the radiomic features and traditional parameters of (18)F-FDG PET with clinical profiles to improve prognostic stratification in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy and surgery. Ann Nuclear Med. 2019;33:657–70.
Yang CK, Yeh JC, Yu WH, et al. Deep convolutional neural network-based positron emission tomography analysis predicts esophageal cancer outcome. J Clin Med. 2019;8:844.
Xie C, Yang P, Zhang X, et al. Subregion-based radiomics analysis for survival prediction in oesophageal tumours treated by definitive concurrent chemoradiotherapy. EbioMedicine. 2019;44:289–97.
Larue R, Klaassen R, Jochems A, et al. Pre-treatment CT radiomics to predict 3-year overall survival following chemoradiotherapy of esophageal cancer. Acta Oncol Stockholm Sweden. 2018;57:1475–81.
Foley KG, Hills RK, Berthon B, et al. Development and validation of a prognostic model incorporating texture analysis derived from standardised segmentation of PET in patients with oesophageal cancer. Eur Radiol. 2018;28:428–36.
Xiong J, Yu W, Ma J, Ren Y, Fu X, Zhao J. The role of PET-based radiomic features in predicting local control of esophageal cancer treated with concurrent chemoradiotherapy. Sci Rep. 2018;8:9902.
Xie C-Y, Hu Y-H, Ho JW-K, et al. Using genomics feature selection method in radiomics pipeline improves prognostication performance in locally advanced esophageal squamous cell carcinoma: a pilot study. Cancers. 2021;13:2145.
Tan X, Ma Z, Yan L, Ye W, Liu Z, Liang C. Radiomics nomogram outperforms size criteria in discriminating lymph node metastasis in resectable esophageal squamous cell carcinoma. Eur Radiol. 2019;29:392–400.
Wu L, Yang X, Cao W, et al. Multiple level CT radiomics features preoperatively predict lymph node metastasis in esophageal cancer: a multicentre retrospective study. Front Oncol. 2019;9:1548.
Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biometr J. 2005;47:458–72.
Hatt M, Vallieres M, Visvikis D, Zwanenburg A. IBSI: an international community radiomics standardization initiative. J Nucl Med. 2018;59(1):287.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Powers DM. Evaluation: from precision, recall and F-measure to ROC, informedness, markedness and correlation. arXiv preprint arXiv:2010.16061. 2020.
Yu J, Ouyang W, Li C, et al. Mapping patterns of metastatic lymph nodes for postoperative radiotherapy in thoracic esophageal squamous cell carcinoma: a recommendation for clinical target volume definition. BMC cancer. 2019;19:927.
Ortiz-Ramon R, Larroza A, Arana E, Moratal D. A radiomics evaluation of 2D and 3D MRI texture features to classify brain metastases from lung cancer and melanoma. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference. 2017. p. 493–6.
Shen C, Liu Z, Guan M, et al. 2D and 3D CT radiomics features prognostic performance comparison in non-small cell lung cancer. Translat Oncol. 2017;10:886–94.
Yang L, Yang J, Zhou X, et al. Development of a radiomics nomogram based on the 2D and 3D CT features to predict the survival of non-small cell lung cancer patients. Eur Radiol. 2019;29:2196–206.
Lubner MG, Stabo N, Lubner SJ, et al. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging. 2015;40:2331–7.
Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer. 1996;78:1820–8.
Ng T, Vezeridis MP. Advances in the surgical treatment of esophageal cancer. J Surg Oncol. 2010;101:725–9.
Pasquali S, Yim G, Vohra RS, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: a network meta-analysis. Ann Surg. 2017;265:481–91.
Tan Z, Ma G, Zhao J, et al. Impact of thoracic recurrent laryngeal node dissection: 508 patients with tri-incisional esophagectomy. J Gastrointest Surg. 2014;18:187–93.
Taniyama Y, Nakamura T, Mitamura A, et al. A strategy for supraclavicular lymph node dissection using recurrent laryngeal nerve lymph node status in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2013;95:1930–7
Yu S, Lin J, Chen C, et al. Recurrent laryngeal nerve lymph node dissection may not be suitable for all early-stage esophageal squamous cell carcinoma patients: an 8-year experience. J Thorac Dis. 2016;8:2803–12.
Sahiner B, Pezeshk A, Hadjiiski LM, et al. Deep learning in medical imaging and radiation therapy. Med Phys. 2019;46:e1-36.
Li H, Han H, Li Z, et al. High-Resolution Chest X-Ray Bone Suppression Using Unpaired CT Structural Priors. IEEE Trans Med Imaging. 2020;39(10):3053–63.
Liao H, Lin W-A, Zhou SK, Luo J. ADN: artifact disentanglement network for unsupervised metal artifact reduction. IEEE Trans Med Imaging. 2019;39:634–43.
Liu M, Chen Y, Fu X, Zhao K, Jiang GL. Proposed revision of CT-based cervical and thoracic lymph node levels for esophageal cancer in UICC 7th version. Radiother Oncol. 2014;113:175–81.
Kim SJ, Pak K, Chang S. Determination of regional lymph node status using (18)F-FDG PET/CT parameters in oesophageal cancer patients: comparison of SUV, volumetric parameters and intratumoral heterogeneity. Br J Radiol. 2016;89:20150673.
Xu M, Wang L, Ouyang M, et al. Prediction of lymph node metastasis by PET/CT metabolic parameters in patients with esophageal squamous cell carcinoma. Nuclear Med Commun. 2019;40:933–9.
Xu Q, Liu ZK, Cao YK, Li YM, Zhu SC. Relationship of gross tumor volume with lymph node metastasis and prognosis of esophageal carcinoma. Zhonghua Zhong Liu Za Zhi. 2012;34(9):684–87.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81402003 and 81972614).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, C., Hu, Y., Han, L. et al. Prediction of Individual Lymph Node Metastatic Status in Esophageal Squamous Cell Carcinoma Using Routine Computed Tomography Imaging: Comparison of Size-Based Measurements and Radiomics-Based Models. Ann Surg Oncol 29, 8117–8126 (2022). https://doi.org/10.1245/s10434-022-12207-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12207-7