Abstract
Objective
The aim of this study was to evaluate whether patients with invasive lobular carcinoma (ILC) are more likely to have discordant clinical and genomic risk than those with invasive ductal carcinoma (IDC) when using the 21-gene recurrence score (RS), and to assess overall survival outcomes of patients with 1–3 positive nodes and RS ≤25 with and without chemotherapy, stratified by histology.
Methods
We performed a cohort study using the National Cancer Database and included patients with hormone receptor-positive, HER2-negative, stage I–III invasive breast cancer who underwent 21-gene RS testing. Our primary outcome was rate of discordant clinical and genomic risk status by histologic subtype. Propensity score matching was used to compare 60-month overall survival in individuals with 1–3 positive nodes and RS ≤25 who did and did not receive chemotherapy.
Results
Overall, 186,867 patients were included in our analysis, including 37,685 (20.2%) patients with ILC. There was a significantly higher rate of discordant clinical and genomic risk in patients with ILC compared with IDC. Among patients with 1–3 positive nodes and RS ≤25, there was no significant difference in survival between those who did and did not receive chemotherapy in the IDC or ILC cohorts. Unadjusted exploratory analyses of patients under age 50 years with 1–3 positive nodes and RS ≤25 showed improved overall survival in IDC patients who received chemotherapy, but not among those with ILC.
Conclusion
Our findings highlight the importance of lobular-specific tools for stratifying clinical and genomic risk, as well as the need for histologic subtype-specific analyses in randomized trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite major advances in the treatment of breast cancer, personalizing therapy to reduce recurrence risk while minimizing harm remains a challenge. Recommendations for treatment such as adjuvant chemotherapy rely on multiple factors, including patient characteristics and overall health status, tumor features, and often molecular assays such as the 70-gene signature or the 21-gene recurrence score (RS).1,2,3 When patient and tumor factors suggest a high risk of recurrence, patients are deemed to have high ‘clinical’ risk and chemotherapy may be considered. Similarly, when molecular assays suggest a high risk of recurrence, tumors are considered to have high ‘genomic’ risk and patients with these high-risk tumors are predicted to benefit from chemotherapy.
However, when clinical and genomic risk are discordant, decisions about the efficacy of chemotherapy become more difficult. In the recently published RxPONDER trial, chemotherapy did not improve invasive disease-free survival in clinically high-risk postmenopausal patients with low genomic risk (based on RS ≤25), although benefit was seen in those who were premenopausal.4 These findings illustrate some of the challenges of treatment selection for those with discordant clinical and genomic risk.
We previously showed that such discordant risk status disproportionately affects patients with invasive lobular carcinoma (ILC) compared with invasive ductal carcinoma (IDC) when genomic risk is evaluated with 70-gene signature testing.5 As the second most common histologic subtype of breast cancer, ILC is thought to have a more indolent course but often presents at higher stages with considerable cumulative risk of late recurrence.6,7,8,9,10,11 We therefore sought to investigate rates of discordant clinical and genomic risk by histologic subtype in patients who underwent RS testing in the National Cancer Database (NCDB).
Specifically, we evaluated the following questions: (1) whether patients with ILC are more likely to have discordant clinical and genomic risk than those with IDC using the 21-gene RS; (2) whether use of chemotherapy differs by histologic subtype stratified by clinical and genomic risk category; and (3) whether patients with 1–3 positive nodes and RS ≤25 have different overall survival outcomes with or without adjuvant chemotherapy stratified by histologic subtype.
Methods
Data Source and Study Cohort
Our study cohort consisted of patients in the NCDB, a national comprehensive clinical surveillance resource that represents over 70% of all newly diagnosed cancer cases in the US.12,13 Participant user files from 2010 to 2016 were used in our analysis, which included patients diagnosed between 2004 and 2016. Due to the de-identified nature of the public-access user files, the study did not require Institutional Review Board approval.
We limited our analysis to patients with invasive hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative tumors with an available 21-gene RS result. Tumors that were estrogen receptor (ER) and/or progesterone receptor (PR)-positive were considered to be HR-positive. We excluded patients with stage IV disease, those who received neoadjuvant therapy, individuals who did not undergo surgery for breast cancer, and those who were missing critical clinical information, including histologic subtype, tumor grade, number of positive lymph nodes on pathology, tumor size, and timing of chemotherapy or endocrine therapy.
Clinical Measures and Outcomes
Our primary outcome was the rate of discordant clinical and genomic risk status by histologic subtype. We assigned clinical risk using a modified Adjuvant! Online calculator as described in the MINDACT trial; we also categorized patients by number of positive lymph nodes on surgical pathology (0, 1–3, or >3 nodes).3,4,14,15,16 The modified Adjuvant! Online risk score includes tumor size, tumor grade, and number of positive lymph nodes identified at surgery; those with a 92% or greater probability of breast cancer-specific survival at 10 years without chemotherapy were deemed to have low clinical risk.17,18 We assigned genomic risk using the 21-gene RS result, with RS >25 considered as high genomic risk and RS ≤25 considered as low genomic risk, consistent with RxPONDER. We defined ‘discordant risk’ as having either clinical high-risk with genomic low-risk status, or clinical low-risk with genomic high-risk status.
Histologic subtype was determined by defined codes in the NCDB. The ILC cohort included codes for ILC or mixed ILC/IDC (histology codes 8520 and 8524 if behavior was invasive). The IDC cohort comprised codes for IDC or invasive mammary carcinoma not otherwise specified (histology codes 8500, 8501, 8502, 8503, and 8523 if behavior was invasive). Menopausal status was approximated using age under 50 years (premenopausal) and over 50 years (postmenopausal) and was included as a predictor in our model. Additionally, the Charlson–Deyo Comorbidity Index was used as a marker of severity of comorbid conditions. The Charlson–Deyo Comorbidity Index is a weighted score from 0 to 3 derived from multiple comorbid conditions, including myocardial infarction, diabetes, and renal disease, with higher score reflecting more comorbid disease.19,20,21
Statistical Analysis
Patient characteristics were described and differences between histologic subtype were evaluated using Chi-square tests for categorical variables and t-tests for continuous variables. Additionally, the frequency of patients in each of the four clinical and genomic risk categories (clinical high/genomic low, clinical low/genomic low, clinical high/genomic high, clinical low/genomic high) was described by histologic subtype, with Chi-square testing used to compare the clinical high/genomic low risk cohort compared with other risk categories combined and across all four risk categories. We also performed subgroup analyses evaluating concordance of genomic and clinical risk by categorical nodal status, histology, and age. Receipt of chemotherapy stratified by histology, nodal status, RS, and age was also assessed. All analyses were prespecified.
Finally, propensity score matching was used to compare 60-month overall survival in individuals who did and did not receive chemotherapy among patients with 1–3 positive nodes and RS ≤25, stratified by histology. A greedy, fixed 1:1 matching method was used and patients were matched by age at diagnosis in years, pathologic stage of disease (stage 2/3 vs. stage 1), and facility type. Weighted matched standardized differences and variance ratios for the propensity score model covariates were used to assess sample balance after matching. Acceptable balance was defined by a maximum of 0.2 for the absolute value of standardized difference and by values within the 0.5–2 range for variance ratio. While we planned a prespecified propensity score-matched analysis evaluating the relationship between chemotherapy and overall survival in the subgroup of women aged under 50 years, with 1–3 positive nodes and RS ≤25, there were too few patients to allow for matching. We therefore performed exploratory analyses evaluating overall survival with and without chemotherapy, using the log-rank test, in this subgroup of women, stratified by histology; we also used the Cox proportional hazards model to evaluate overall survival both unadjusted and adjusted for Charlson–Deyo Comorbidity Index.
Hypothesis tests were two-sided and the significance threshold was set to 0.05. Statistical analyses were performed using Stata 16 (StataCorp LLC, College Station, TX, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
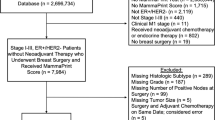
A total of 2,696,734 patients with breast cancer were included in the original NCDB database. After excluding patients based on the criteria previously described, there were 186,867 patients with stage I–III, HR-positive, HER2-negative disease without neoadjuvant therapy who underwent breast surgery and received 21-gene RS testing who were included in our analysis (Fig. 1). Of these, 149,182 (79.8%) patients had IDC and 37,685 (20.2%) patients had ILC. Median follow-up time for the cohort was 36 months (IQR 23.1–59.0). The majority of patients had ER- and PR-positive cancers (n = 169,337, 90.6%), with 17,320 (9.27%) patients having ER-positive and PR-negative cancers.
Invasive Lobular Carcinoma Versus Invasive Ductal Carcinoma Cohorts
Patients in the ILC cohort were slightly older than those in the IDC cohort (mean age 60.5 years vs. 58.9; p < 0.001). Additionally, they were more likely to present with higher stage disease and underwent mastectomy at higher rates (Table 1). The ILC tumors were significantly less likely to be grade 3, and overall chemotherapy was used less often in the ILC cohort compared with those with IDC. There was also a statistically significant difference in Charlson–Deyo score between the ILC and IDC cohorts, with ILC patients more likely to have a Charlson–Deyo score of 0 compared with those with IDC (85.0% vs. 84.5%, p = 0.015).
Clinical and Genomic Risk Discordance
We found a significantly higher rate of clinical high/genomic low risk status in patients with ILC compared with IDC. Patients with ILC were more likely to have high clinical risk by modified Adjuvant! Online than those with IDC (43% vs. 35.6%; p < 0.001) [Table 1]. Consistent with this finding, those with ILC were less likely to be node-negative than those with IDC (80.7% vs. 82.8%; p < 0.001). While clinical risk was higher in ILC patients, genomic risk by RS was much lower, with an incidence of RS >25 in ILC patients of 8.4% compared with 16.2% in IDC patients (p < 0.001). Together, this resulted in significantly higher rates of this discordant risk category, with 37.8% of the ILC group being clinical high/genomic low compared with 24.9% of the IDC group (p < 0.001) [Table 2].
Within the group of patients with 1–3 positive nodes, patients with ILC were significantly more likely to have an RS ≤25 compared with those with IDC (92.5% vs. 85.8%, respectively; p < 0.001) [Table 3]. This difference was more pronounced in those with >3 positive nodes, with the vast majority of ILC patients in this group (90.1%) having an RS ≤25 compared with 78.3% in the IDC group (p < 0.001) [Table 3]. Interestingly, among patients under the age of 50 years, ILC patients remained significantly more likely to have RS ≤25 than those with IDC across all nodal involvement categories. In patients under age 50 years with 1–3 positive nodes, low RS occurred in 94.8% of the ILC group versus 85.1% of the IDC group; among the small number of patients with >3 positive nodes, low RS occurred in 94.7% of the ILC group versus 70.6% of the IDC group (both p < 0.001 [Table 3].
Chemotherapy Use
We then evaluated the receipt of chemotherapy by histology, nodal status, RS category, and age. Among patients with RS ≤25, there was no difference in receipt of chemotherapy by histology across the node-negative, 1–3 positive nodes, and >3 positive nodal groups (Fig. 2). However, among patients with RS >25, those with ILC were significantly less likely to receive chemotherapy than those with IDC among patients with negative nodes or 1–3 positive nodes (both p < 0.001). In those with high clinical risk and RS >25, chemotherapy was used significantly more often in patients under 50 years of age compared with those aged 50 years or older, in both the ILC and IDC groups. However, among clinically high-risk patients with RS >25 and aged over 50 years, those with ILC were still less likely to receive chemotherapy than those with IDC (63.3% vs. 74.8%, p < 0.001). In ILC patients with high clinical risk and RS >25, 88.7% of patients were aged 50 years or older and 63.3% received chemotherapy compared with 80.5% of those under age 50 years (p < 0.001). Among IDC patients with high clinical risk and RS >25, 78.2% of patients were aged 50 years or older and 74.8% received chemotherapy compared with 88.2% of those under age 50 years (p < 0.001).
Proportion of patients who received chemotherapy, by histology, nodal status, and recurrence score. Chi-square test comparing patients with ILC/RS >25/node-negative vs. IDC/RS >25/node-negative and ILC/RS >25/1–3 positive nodes versus IDC/RS >25/1–3 positive nodes both yielded p < 0.001. All other comparisons were not statistically significant. ILC invasive lobular carcinoma, IDC invasive ductal carcinoma, RS recurrence score
Overall Survival Outcomes
Finally, we performed a propensity score-matched analysis to compare overall survival in individuals who received chemotherapy compared with those who did not, by histology among those with 1–3 positive nodes and RS ≤25, including patients of all ages. All covariates used in matching met the sample balance criteria, and baseline clinical and pathologic characteristics of the matched cohorts are provided in electronic supplementary Table 1. Among patients with IDC, there was no statistically significant difference in survival between those who did and did not receive chemotherapy (stratified log-rank test p = 0.278) [Fig. 3a]. Similarly, for patients with ILC, survival between those who received chemotherapy and those who did not was not significantly different from one another (stratified log-rank test p = 0.532) [Fig. 3b]. There were too few patients under the age of 50 years to perform a propensity score-matched analysis, therefore exploratory results from unmatched survival analyses are reported. For women under age 50 years, with 1–3 positive nodes and RS ≤25, unadjusted analysis showed that chemotherapy was associated with a significant improvement in overall survival for those with IDC (hazard ratio [HR] 0.44, 95% confidence interval [CI] 0.22–0.86; p = 0.016) but not for those with ILC (HR 0.54, 95% CI 0.14–2.18; p = 0.39). The association between chemotherapy and improved overall survival in those with IDC persisted when adjusted for Charlson–Deyo Index (HR 0.44, 95% CI 0.22–0.85; p = 0.016). There was no statistical interaction between chemotherapy and histology on overall survival.
Propensity score-matched analysis using the greedy fixed matching method comparing overall survival in individuals who received chemotherapy compared with those who did not among patients with 1–3 positive nodes and RS ≤25, by histology. a For patients with IDC, survival between those who received chemotherapy and those who did not was not significantly different from one another (stratified log-rank test p = 0.278). b Similarly, for patients with ILC, survival between those who received chemotherapy and those who did not was not significantly different from one another (stratified log-rank test p = 0.532). ILC invasive lobular carcinoma, IDC invasive ductal carcinoma, RS recurrence score
Discussion
In this study of 186,867 patients with HR-positive, HER2-negative invasive breast cancer who underwent 21-gene RS testing in the NCDB, we found that patients with ILC have higher rates of discordant clinical and genomic risk than those with IDC. This finding is consistent with our prior work, which showed higher rates of discordant clinical and genomic risk in ILC patients using the 70-gene signature.5 Additionally, we found that ILC patients with 1–3 positive nodes were significantly more likely to have RS ≤25 compared with those with IDC, in both those under age 50 years and those aged 50 years or older.
Among those with RS ≤25, there was no difference in receipt of chemotherapy by histology regardless of nodal involvement. However, among those with RS >25, those with ILC were significantly less likely to receive chemotherapy than those with IDC among patients with negative nodes or 1–3 positive nodes. While these findings are consistent with our prior study, which showed lower rates of chemotherapy use in patients with high clinical risk ILC and high genomic risk as defined by 70-gene signature testing, they are nonetheless surprising given the general acceptance of chemotherapy in the setting of RS >25 and did not appear to be driven by older age in the ILC group.5,14 These findings may reflect hesitation on the part of clinicians and patients to utilize chemotherapy in ILC, where multiple studies have shown less benefit in the neoadjuvant and adjuvant settings.22,23 Together, this illustrates the treatment dilemma that clinicians and patients face. While patients with ILC are more likely to have high clinical risk, which portends increased risk of recurrence without chemotherapy, genomic assays and reported series suggest decreased chemotherapy benefit.24
We did not demonstrate any effect of chemotherapy on overall survival among patients with 1–3 positive nodes and RS ≤25 in our propensity score-matched analyses, regardless of histologic subtype. Interestingly, we did find an association between chemotherapy and overall survival in unmatched analysis of IDC patients under age 50 years with 1–3 positive nodes and RS ≤25, but not among those under age 50 years with ILC. Given the retrospective nature of this analysis, differences in patient selection for receiving chemotherapy likely contribute to chemotherapy-related outcomes. While we attempted to account for potential confounders in treatment selection by using propensity score matching by age at diagnosis, pathologic stage, and facility type, it is unlikely that we were able to fully adjust for potential bias between the chemotherapy and non-chemotherapy groups. Additionally, there may be a subgroup of patients with ILC within this cohort who may indeed benefit from chemotherapy. As such, this question should remain open and more research is needed.
This bias is greater in our exploratory unmatched analysis in patients under age 50 years, which showed a significant improvement in overall survival among IDC patients who received chemotherapy compared with those who did not, but no difference in those with ILC. There are several potential explanations for the findings of our exploratory analyses. One possibility is that among those under age 50 years with 1–3 positive nodes and RS ≤25, the overall survival difference observed in the IDC group resulted from patient selection bias and not chemotherapy effect. Another explanation is that we were unable to detect an overall survival benefit in ILC patients under age 50 years, with 1–3 positive nodes and RS ≤25 because of small numbers in this subgroup. Lastly, it is possible that chemotherapy improves overall survival in IDC patients but not ILC patients under the age of 50 years with 1–3 positive nodes and RS ≤25; for this reason, reporting of long-term and overall survival outcomes by histologic subtype from trials such as RxPONDER is needed.
While the RxPONDER trial showed a significant improvement in invasive disease-free survival in patients under age 50 years who had 1–3 positive nodes and RS ≤25 who received chemotherapy, we cannot directly compare our results since the NCDB lacks recurrence endpoints. It is important to note that for patients with HR-positive/HER2-negative tumors, and ILC especially, recurrence events and consequently impact on overall survival can happen at later timepoints, highlighting the need for longer term follow-up.25,26 Additionally, we lacked data on type of endocrine therapy, which likely impacts outcomes. Finally, other limitations of our study include lack of information regarding type of chemotherapy regimen, adherence to endocrine therapy, and short follow-up time for patients included in our study.
This study has many strengths, including the use of a relatively large number of ILC patients, and is now the second study to demonstrate that patients with ILC have high rates of discordance between clinical and genomic risk based on widely used molecular assays. While many studies show that ILC tumors have lower response rates to chemotherapy in the neoadjuvant setting, and less benefit from chemotherapy in the adjuvant setting, there may still be a subset of chemotherapy-sensitive ILC cases. Recent work has identified genomic signatures that identify subtypes within ILC, suggesting heterogeneity within this tumor type.27,28 The high incidence of high clinical risk among patients with ILC highlights the need for both more effective therapies, and potentially ILC-specific prediction tools. More broadly, improving outcomes for these patients with ILC will require not only equitable enrollment of ILC patients into breast cancer clinical trials but also histologic subtype-specific reporting of trial results.
Data Availability
The data that support the findings of this study are available from the NCDB, but restrictions apply to the availability of these data, which were used under license for the current study and therefore are not publicly available. Data are however available from the authors upon reasonable request and with permission of the NCDB.
References
Van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. https://doi.org/10.1038/415530a.
van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. New England J Med. 2002;347:1999–2009. https://doi.org/10.1056/nejmoa021967.
Cardoso F, van’t Veer L, Poncet C, et al. MINDACT: long-term results of the large prospective trial testing the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy in breast cancer patients. J Clin Oncol. 2020. https://doi.org/10.1200/JCO.2020.38.15_suppl.506.
Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. New England J Med. 2021;385:2336–47. https://doi.org/10.1056/NEJMOA2108873.
Abel MK, Shui AM, Melisko M, et al. The incidence of discordant clinical and genomic risk in patients with invasive lobular or ductal carcinoma of the breast: a National Cancer Database Study. NPJ Breast Cancer. 2021;7:156. https://doi.org/10.1038/S41523-021-00366-X.
Sledge GW, Chagpar A, Perou C. Collective wisdom: lobular carcinoma of the breast. Am Soci Clin Oncol Educat Book. 2016;35:18–21.
Mamtani A, King TA. Lobular breast cancer: different disease, different algorithms? Surg Oncol Clin North America. 2018;27:81–94. https://doi.org/10.1016/j.soc.2017.07.005.
Lips EH, Mukhtar RA, Yau C, et al. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Can Res Treat. 2012;136:35–43. https://doi.org/10.1007/s10549-012-2233-z.
Rakha EA, El-Sayed ME, Powe DG, et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Can. 2008;44:73–83. https://doi.org/10.1016/j.ejca.2007.10.009.
Adachi Y, Ishiguro J, Kotani H, et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Can. 2016. https://doi.org/10.1186/s12885-016-2275-4.
Thomas M, Kelly ED, Abraham J, Kruse M. Invasive lobular breast cancer: a review of pathogenesis, diagnosis, management, and future directions of early stage disease. Semin Oncol. 2019;46:121–32. https://doi.org/10.1053/j.seminoncol.2019.03.002.
Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the national cancer data base. J Clin Oncol. 2009;27:4177–81. https://doi.org/10.1200/JCO.2008.21.7018.
Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research a review. JAMA Oncol. 2017;3:1722–8. https://doi.org/10.1001/jamaoncol.2016.6905.
Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. New England J Med. 2018;379:111–21. https://doi.org/10.1056/NEJMOA1804710.
Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–25. https://doi.org/10.1200/JCO.2005.06.178.
Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–91. https://doi.org/10.1200/JCO.2001.19.4.980.
Mook S, Schmidt MK, Rutgers EJ, et al. Calibration and discriminatory accuracy of prognosis calculation for breast cancer with the online Adjuvant! program: a hospital-based retrospective cohort study. Lancet Oncol. 2009;10:1070–6. https://doi.org/10.1016/S1470-2045(09)70254-2.
Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. New England J Med. 2019;380:2395–405. https://doi.org/10.1056/NEJMOA1904819/SUPPL_FILE/NEJMOA1904819_DATA-SHARING.PDF.
National Cancer Database Participant User File, 2018 Data Dictionary. Available at: https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/puf_data_dictionary.ashx. Accessed 1 Jan 2022
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. https://doi.org/10.1016/0895-4356(92)90133-8.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Diseas. 1987;40:373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Marmor S, Hui JYC, Huang JL, et al. Relative effectiveness of adjuvant chemotherapy for invasive lobular compared with invasive ductal carcinoma of the breast. Cancer. 2017;123:3015–21. https://doi.org/10.1002/cncr.30699.
Tamirisa N, Williamson HV, Thomas SM, et al. The impact of chemotherapy sequence on survival in node-positive invasive lobular carcinoma. J Surg Oncology. 2019;120:132–41.
Trapani D, Gandini S, Corti C, et al. Benefit of adjuvant chemotherapy in patients with lobular breast cancer: a systematic review of the literature and metanalysis. Can Treat Rev. 2021;97:102205. https://doi.org/10.1016/J.CTRV.2021.102205.
Dowsett M, Sestak I, Regan MM, et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol. 2018;36:1941–8. https://doi.org/10.1200/JCO.2017.76.4258.
Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 Years. New England J Med. 2017;377:1836–46. https://doi.org/10.1056/NEJMOA1701830/SUPPL_FILE/NEJMOA1701830_DISCLOSURES.PDF.
McCart Reed AE, Lal S, Kutasovic JR, et al. LobSig is a multigene predictor of outcome in invasive lobular carcinoma. NPJ Breast Cancer. 2019;5:18. https://doi.org/10.1038/s41523-019-0113-y.
Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–19. https://doi.org/10.1016/J.CELL.2015.09.033.
Acknowledgment
Amy M. Shui is part of the Biostatistics Core that is generously supported by the University of California San Francisco Department of Surgery.
Funding
Rita A. Mukhtar is supported by National Cancer Institute Award K08CA256047. All other authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MKA, AMS, and RAM. The first draft of the manuscript was written by MKA and RAM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosure
A. Jo Chien receives research funding from Merck, Puma, Amgen, and Seattle Genetics. Hope S. Rugo receives research support for clinical trials through the University of California from Pfizer, Merck, Novartis, Lilly, Roche, Odonate, Daiichi, Seattle Genetics, Macrogenics, Sermonix, Astra Zeneca, OBI, Gilead, and Ayala, and has also received honoraria from Puma, Samsung, and Napo. Michelle Melisko receives research funding from Astra Zeneca, Novartis, KCRN Research, and Puma, and consulting fees from Biotheranostics. Frederick Baehner is the Chief Medical Officer of Precision Oncology at Exact Sciences. Mary Kathryn Abel, Amy M. Shui, and Rita A. Mukhtar declare no competing interests.
Ethical Approval
Our study was deemed exempt by the University of California San Francisco Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abel, M.K., Shui, A.M., Chien, A.J. et al. The 21-Gene Recurrence Score in Clinically High-Risk Lobular and Ductal Breast Cancer: A National Cancer Database Study. Ann Surg Oncol 29, 7739–7747 (2022). https://doi.org/10.1245/s10434-022-12065-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12065-3