Abstract
Background
The prognostic significance of peritoneal lavage cytology (PLC) in patients with pancreatic ductal adenocarcinoma (PDAC) remains controversial. The purpose of this study was to evaluate the prognostic impact of PLC status in PDAC patients.
Methods
Patients intending to undergo resection for PDAC between 2007 and 2020 were included. Survival was compared among patients who underwent resection with negative or positive PLC status and those who did not undergo resection. Univariable and multivariable analyses were conducted to evaluate the prognostic impact of positive PLC status. A systematic literature review was performed to evaluate the correlation between prognosis and the positive PLC rate.
Results
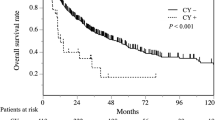
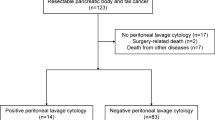
A total of 480 patients formed the study cohort and were divided as follows: 438 in the negative PLC group, 18 in the positive PLC group, and 24 in the no resection group. Although the median survival time significantly differed between the negative and positive PLC groups (35.7 vs. 13.6 months, P < 0.001), it did not significantly differ between the positive PLC and no resection groups (13.6 vs. 12.2 months, P = 0.605). Multivariable analyses demonstrated that positive PLC status (hazard ratio = 3.54, 95% confidence interval = 1.97–6.38, P < 0.001) was the strongest poor prognostic factor. Based on statistical analyses for the systematic review, the prognostic impact of positive PLC status weakened significantly as the institutional positive PLC rate increased (P = 0.044).
Conclusions
Resection did not improve the prognosis of patients with positive PLC status in our cohort. The institutional positive PLC rate may be a good reference for surgical indication in these patients.




Similar content being viewed by others
References
Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152(3 Suppl 1):S43–9.
Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836–47.
Picozzi VJ, Oh SY, Edwards A, et al. Five-year actual overall survival in resected pancreatic cancer: a contemporary single-institution experience from a multidisciplinary perspective. Ann Surg Oncol. 2017;24(6):1722–30.
Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801–10.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Hishinuma S, Ogata Y, Tomikawa M, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10(4):511–8.
Amin MES, Greene F, Byrd D. The AJCC TNM cancer staging manual. 8th edn. New York: Springer; 2017.
Brierley JDGM, Wittekind C. TNM classification of malignant tumours. 8th edn. New York: Wiley; 2017.
Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg. 1991;161(1):26–30.
Meszoely IM, Lee JS, Watson JC, et al. Peritoneal cytology in patients with potentially resectable adenocarcinoma of the pancreas. Am Surg. 2004;70(3):208–14.
Ferrone CR, Haas B, Tang L, et al. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg. 2006;10(10):1347–53.
Yoshioka R, Saiura A, Koga R, et al. The implications of positive peritoneal lavage cytology in potentially resectable pancreatic cancer. World J Surg. 2012;36(9):2187–91.
Yamada S, Fujii T, Kanda M, et al. Value of peritoneal cytology in potentially resectable pancreatic cancer. Br J Surg. 2013;100(13):1791–6.
Satoi S, Murakami Y, Motoi F, et al. Reappraisal of peritoneal washing cytology in 984 patients with pancreatic ductal adenocarcinoma who underwent margin-negative resection. J Gastrointest Surg. 2015;19(1):6–14.
Abe T, Ohuchida K, Endo S, et al. Clinical importance of intraoperative peritoneal cytology in patients with pancreatic cancer. Surgery. 2017;161(4):951–8.
Hoshimoto S, Hishinuma S, Shirakawa H, et al. Prognostic significance of intraoperative peritoneal washing cytology for patients with potentially resectable pancreatic ductal adenocarcinoma. Pancreatology. 2017;17(1):109–14.
Tsuchida H, Fujii T, Mizuma M, et al. Prognostic importance of peritoneal washing cytology in patients with otherwise resectable pancreatic ductal adenocarcinoma who underwent pancreatectomy: a nationwide, cancer registry–based study from the Japan Pancreas Society. Surgery. 2019;166(6):997–1003.
Aoki S, Mizuma M, Hayashi H, et al. Prognostic impact of intraoperative peritoneal cytology after neoadjuvant therapy for potentially resectable pancreatic cancer. Pancreatology. 2020;20(8):1711–7.
Sakoda T, Uemura K, Kondo N, et al. Prognostic value of peritoneal lavage cytology in patients with pancreatic ductal adenocarcinoma stratified by the resectability status. J Gastrointest Surg. 2021. https://doi.org/10.1007/s11605-021-04978-3.
Kubo M, Sakamoto M, Fukushima N, et al. Less aggressive features of colorectal cancer with liver metastases showing macroscopic intrabiliary extension. Pathol Int. 2002;52(8):514–8.
Yachida S, Fukushima N, Sakamoto M, et al. Implications of peritoneal washing cytology in patients with potentially resectable pancreatic cancer. Br J Surg. 2002;89(5):573–8.
Yamada S, Takeda S, Fujii T, et al. Clinical implications of peritoneal cytology in potentially resectable pancreatic cancer: positive peritoneal cytology may not confer an adverse prognosis. Ann Surg. 2007;246(2):254–8.
Takadate T, Morikawa T, Ishida M, et al. Staging laparoscopy is mandatory for the treatment of pancreatic cancer to avoid missing radiologically negative metastases. Surg Today. 2021;51(5):686–94.
Hirabayashi K, Imoto A, Yamada M, et al. Positive intraoperative peritoneal lavage cytology is a negative prognostic factor in pancreatic ductal adenocarcinoma: a retrospective single-center study. Front Oncol. 2015;5:182.
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(8):1028–61.
Society JP. General rules for the study of pancreatic cancer. 7th edn. Tokyo: Kanehara & Co.; 2016.
Wittekind CBJ, Lee A, Eychen EV. TNM Supplement: a Commentary on Uniform Use. 5th edn. Hoboken: Wiley, New York; 2019.
Unno M, Motoi F, Matsuyama Y, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol. 2019;37(4_suppl):189.
Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93(10):1232–7.
Hank T, Hinz U, Tarantino I, et al. Validation of at least 1 mm as cut-off for resection margins for pancreatic adenocarcinoma of the body and tail. Br J Surg. 2018;105(9):1171–81.
Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993.
Suenaga M, Fujii T, Yamada S, et al. Peritoneal lavage tumor DNA as a novel biomarker for predicting peritoneal recurrence in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2021;28(4):2277–86.
Virgilio E, Giarnieri E, Giovagnoli MR, et al. Gastric cancer cells in peritoneal lavage fluid: a systematic review comparing cytological with molecular detection for diagnosis of peritoneal metastases and prediction of peritoneal recurrences. Anticancer Res. 2018;38(3):1255–62.
Subbarayan D, Bhattacharya J, Rani P, et al. Use of panel of markers in serous effusion to distinguish reactive mesothelial cells from adenocarcinoma. J Cytol. 2019;36(1):28–31.
Motherby H, Nadjari B, Friegel P, et al. Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999;20(6):350–7.
Mohanty SK, Dey P. Serous effusions: diagnosis of malignancy beyond cytomorphology. An analytic review. Postgrad Med J. 2003;79(936):569–74.
Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer. 2002;5(2):69–76.
Hata T, Mizuma M, Iseki M, et al. Circulating tumor DNA as a predictive marker for occult metastases in pancreatic cancer patients with radiographically non-metastatic disease. J Hepatobiliary Pancreat Sci. 2021;28(8):648–58.
Sergeant G, Roskams T, van Pelt J, et al. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer. 2011;11:47.
Kodera Y, Nakanishi H, Ito S, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase-polymerase chain reaction after 5 years of follow-up. J Am Coll Surg. 2006;202(2):231–6.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study conception and design: SK, YS, KM. Acquisition of data: SK, HF, YS, AO. Analysis and interpretation of data: SK, KM, SN, MO. Drafting of manuscript: SK, YS, KM, WH. Critical revision: MN, KH, SI, KK, TA, KM, YI, TK, EH. All authors gave final approval of the version to be submitted.
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Subgroup analysis for overall survival by postoperative chemotherapy between the positive PLC and no resection groups. a FOLFIRINOX or GNP therapy. b Other chemotherapy regimens. FOLFIRINOX 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan; GNP gemcitabine and nab-paclitaxel (TIF 4014 kb)
Rights and permissions
About this article
Cite this article
Kawakatsu, S., Shimizu, Y., Natsume, S. et al. Prognostic Significance of Intraoperative Peritoneal Lavage Cytology in Patients with Pancreatic Ductal Adenocarcinoma: A Single-Center Experience and Systematic Review of the Literature. Ann Surg Oncol 29, 5972–5983 (2022). https://doi.org/10.1245/s10434-022-11722-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11722-x